 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Muscone
Premium Synthetic Ingredient for Perfumery
Muscone is a synthetic macrocyclic ketone that replicates the primary scent molecule found in natural musk from the musk deer. It delivers a soft, sweet, and exceptionally persistent musky odor, devoid of animalic harshness. Functionally, Muscone acts as a superior fixative and musky base note, often used to enhance diffusion, add elegance, and replace natural musk components in perfumery.
Premium Synthetic Ingredient for Perfumery
Muscone is a synthetic macrocyclic ketone that replicates the primary scent molecule found in natural musk from the musk deer. It delivers a soft, sweet, and exceptionally persistent musky odor, devoid of animalic harshness. Functionally, Muscone acts as a superior fixative and musky base note, often used to enhance diffusion, add elegance, and replace natural musk components in perfumery.
Premium Synthetic Ingredient for Perfumery
Muscone is a synthetic macrocyclic ketone that replicates the primary scent molecule found in natural musk from the musk deer. It delivers a soft, sweet, and exceptionally persistent musky odor, devoid of animalic harshness. Functionally, Muscone acts as a superior fixative and musky base note, often used to enhance diffusion, add elegance, and replace natural musk components in perfumery.
Technical Ingredient Overview
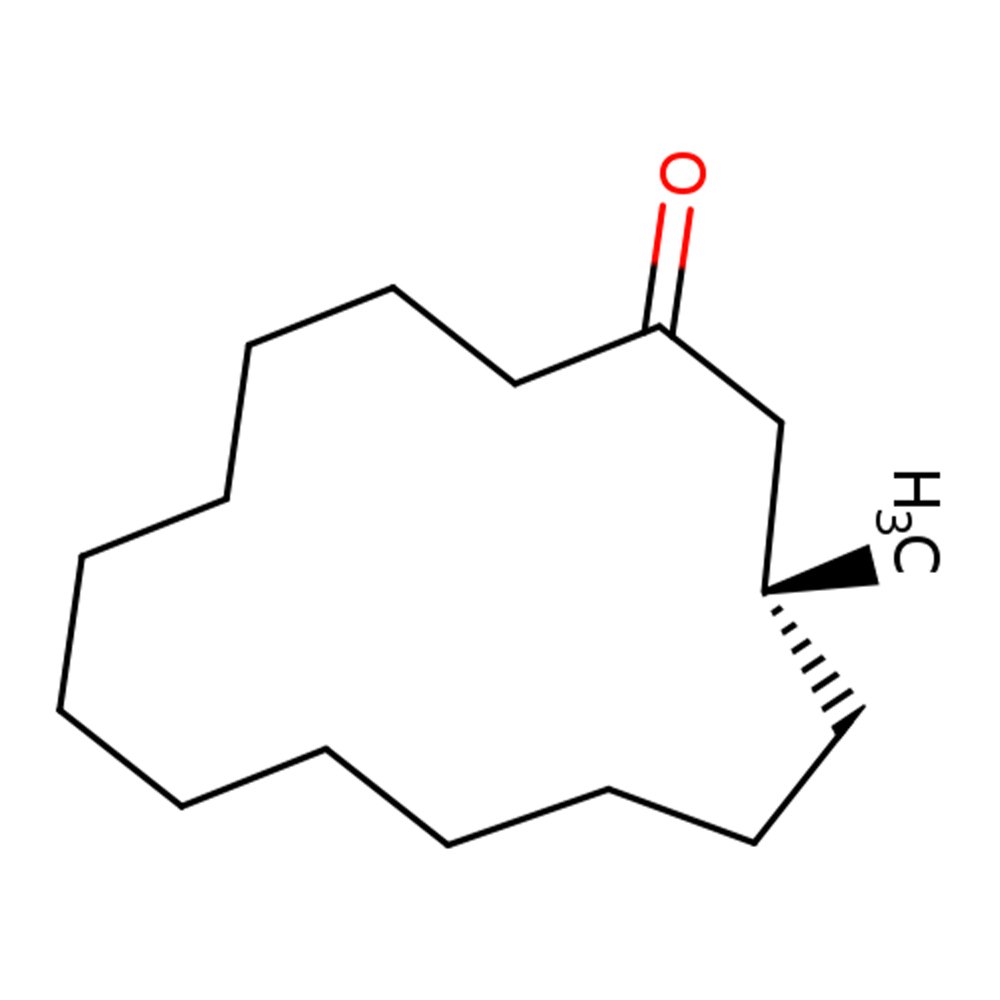
🔎 Chemical Name — 3-Methylcyclopentadecanone
🧪 Synonyms — 3-Methylcyclopentadecanone, Moschus ketone, Methyl Exaltone, Muskone
📂 CAS — 541-91-3
📘 FEMA — 3434
⚖️ MW — 238.4 g/mol
📝 Odor Type — Musk
📈 Odor Strength — Not specified
👃🏼 Odor Profile — Sweet, musky, animalic, powdery, fatty, natural
⚗️ Uses — Fine fragrance, shampoo, shower gel, soap
🧴 Appearance — Colorless to almost colorless clear liquid
What is Muscone?
Muscone is a macrocyclic ketone and the primary odoriferous constituent of natural musk, originally derived from the musk deer (Moschus moschiferus). Due to ethical and sustainability concerns, the harvesting of natural musk has been largely replaced by synthetic production methods. Today, Muscone is primarily produced synthetically, offering an ethical and sustainable alternative to natural musk (The Allure of Musk, n.d.). It is recognized for its soft, sweet, and warm animalic scent, rendering it a valuable ingredient in perfumery, embodying the classic, rich musk character.
Historical Background
Muscone was isolated by the German chemist Heinrich Walbaum in 1906 as the principal odoriferous component of natural musk (Behr & Neff, 2013). However, its complex macrocyclic structure, which was highly unusual for the period, was not fully elucidated until 1926 by Leopold Ruzicka (Ruzicka, 1926). Ruzicka's pioneering investigations into macrocyclic compounds, including muscone, fundamentally expanded the understanding of organic chemistry and challenged the prevailing Baeyer strain theory, which predicted the instability of large-ring structures (Baeyer, 1885). This groundbreaking work earned him a Nobel Prize in Chemistry in 1939. The subsequent development of industrial-scale synthetic pathways for Muscone marked a significant milestone, allowing for its widespread use in the fragrance industry (Kraft et al., 2000).
Olfactory Profile
Muscone is categorized within the macrocyclic musk scent family, a class of large-ring compounds renowned for their intricate and often subtle olfactory characteristics. Its primary odor is defined by a soft, sweet, highly diffusive musky aroma complemented by distinct warm, animalic undertones (Agoratopia, n.d.; The Perfumers Apprentice, n.d.). Beyond these foundational descriptors, Muscone exhibits a multifaceted character:
Sweet & Powdery: It imparts a delicate sweetness, frequently described as powdery or cosmetic, contributing to an overall perception of softness and elegance.
Animalic & Fatty: A subtle yet essential animalic facet provides depth and warmth, reminiscent of natural skin or fur, without being excessively heavy or fecal. It also presents a slight fatty or waxy nuance.
Velvety & Round: Muscone serves to "round out" and harmonize compositions, imparting a velvety texture to the overall fragrance. It functions as a unifying element between disparate notes, facilitating a seamless blend.
Intensity & Tenacity: Possessing moderate to high odor strength, Muscone demonstrates exceptional tenacity, establishing it as a potent base note material. Its scent evolution is gradual yet persistent, providing an enduring foundation for fragrance compositions.
Volatility & Fixative Role: Despite its high molecular weight and low volatility, Muscone exhibits notable diffusion for a musk. It operates primarily as a base note and an excellent fixative, effectively extending the presence of more volatile top and heart notes and contributing significantly to the overall longevity and sillage of a perfume. Its capacity to subtly influence the entire fragrance pyramid from its foundation renders it an indispensable tool in perfumery.
Applications in Fine Fragrance
Within fine fragrance applications, Muscone functions as a cornerstone ingredient, highly valued for its ability to impart a luxurious and sensual warmth. Its versatility permits its incorporation into a diverse array of fragrance types and accords:
Role in Compositions: Muscone serves as a fundamental base note, anchoring lighter components and providing substantial longevity and diffusion. It contributes to the overall elegance and sophistication of a perfume. Its soft, warm, and subtly animalic character renders it ideal for enhancing skin-like nuances and contributing a "second skin" effect.
Typical Accords:
Floral Accords: It seamlessly complements white florals (e.g., jasmine, tuberose, gardenia) by adding a creamy, sensual depth and a touch of warmth, thereby mitigating any potential for excessive sharpness or heaviness. It also integrates effectively with rose and other classic floral notes.
Oriental Accords: Muscone is indispensable in oriental and amber compositions, where its animalic and sweet facets meld harmoniously with resins, balsams, and spices, contributing to their opulent and enduring character.
Woody Accords: It enhances woody notes (e.g., sandalwood, cedarwood, patchouli) by introducing richness, smoothness, and a subtle animalic facet that imbues warmth and naturalness.
Powdery/Aldehydic Accords: Its intrinsic powdery quality renders it a natural complement for classic aldehydic and powdery fragrances, contributing to their clean yet deep, sophisticated character.
Pairing Behavior: Muscone exhibits exceptional compatibility with other musks, facilitating the creation of complex and multi-layered musk accords. It also synergizes effectively with Ambroxan, various woods, vanilla, and ionones, amplifying their richness and diffusion. Its capacity to "lift" and "round out" other notes, thereby providing a seamless transition between them, represents a primary reason for its extensive utilization.
Performance in Formula
Muscone consistently demonstrates excellent performance characteristics within fragrance formulations:
Behavior in Blends: It is highly stable and miscible with the majority of fragrance raw materials, ensuring effortless incorporation into diverse formulations without issues of discoloration or degradation. Its subtle yet powerful impact ensures seamless integration, enhancing rather than dominating other notes.
Diffusion: Despite its high molecular weight and relatively low volatility, Muscone is recognized for its remarkable diffusion. It contributes significantly to the sillage (the olfactive trail left by a fragrance wearer), enabling effective projection of the fragrance while maintaining its intimate warmth.
Impact: Muscone exerts a profound impact even at low concentrations, imparting depth, warmth, and a sensual quality. It functions as an "invisible" enhancer, optimizing the overall balance and richness of a composition.
Fixative Strength: As a macrocyclic musk, Muscone is an outstanding fixative. It effectively diminishes the evaporation rate of more volatile components, thereby extending the overall longevity of the fragrance and ensuring its persistence on the skin for extended durations.
Compatibility: It exhibits broad compatibility across various perfumery bases, including alcoholic solutions, emulsions (e.g., creams, lotions), and anhydrous systems (e.g., oils).
Industrial & Technical Uses
Beyond its prevalent application in fine fragrances and personal care products (such as shampoos, shower gels, and soaps, where it imparts lasting freshness and a luxurious feel), Muscone has been explored in specialized technical applications. Ongoing research into its derivatives and analogs continues, particularly in the domains of chemical synthesis and the study of olfaction. For instance, investigations have explored the preparation and characterization of Muscone-based delivery systems, illustrating its potential in controlled release applications (Yan et al., 2024).
Regulatory & Safety Overview
IFRA Status: Muscone adheres to the standards set forth by the International Fragrance Association (IFRA), which regulates the safe use of fragrance ingredients globally. It is listed without specific restrictions beyond good manufacturing practices in numerous applications, reflecting its generally favorable safety profile. Users are advised to consult the latest IFRA Standards, typically found in Amendment 48 onwards, for specific concentration limits pertinent to various product categories.
GHS Classification: Based on available Safety Data Sheets (SDS), Muscone is generally not classified as hazardous under the OSHA Hazard Communication Standard (The Perfumers Apprentice, n.d.).
EU Cosmetics Regulation: Within the European Union, Muscone is authorized for use in cosmetic products. Consistent with all fragrance ingredients, its application must conform to Regulation (EC) No 1223/2009 on cosmetic products, which ensures consumer safety. Synthetic musk odorants are permissible, with specific restrictions primarily targeting older nitro musks (Publishers Panel, n.d.).
Toxicology: Toxicological assessments indicate that Muscone possesses low acute oral and dermal toxicity. While it may act as a mild skin irritant in concentrated forms, similar to numerous fragrance raw materials, it is generally well-tolerated at typical usage levels in finished products (ChemicalBook, n.d.). The potential for sensitization is considered low.
FEMA: 3434. This FEMA GRAS (Generally Recognized As Safe) designation pertains to its potential use as a flavoring agent, which often correlates with a favorable safety profile for dermal applications.
Comparative Analyses: Muscone vs. Other Key Musks
To fully appreciate Muscone's distinctive position, a comparative analysis with other prominent musk types frequently employed in perfumery is beneficial:
Muscone (Macrocyclic):
Profile: Characterized by a rich, animalic, sweet, fatty, powdery, and subtly natural "skin" musk aroma. It provides excellent radiance and depth, being considered one of the closest synthetic approximations to natural musk.
Performance: Exhibits exceptional tenacity and diffusion. Functions as a superb fixative, imparting warmth and rounding effects to compositions.
Advantages: High quality, naturalistic character, robust fixative properties.
Limitations: May be relatively more expensive compared to other synthetic musk types.
Exaltolide (Macrocyclic - Firmenich):
Profile: Possesses a very clean, subtle, transparent, powdery, waxy, sweet, and slightly fruity (cherry/raspberry nuance) scent. It is less animalic than Muscone.
Performance: Offers excellent diffusion and tenacity, creating an elegant "second skin" effect. Frequently utilized for its sophisticated transparency.
Comparison to Muscone: Both are macrocyclics and effective fixatives; however, Exaltolide is considerably cleaner and less animalic. Muscone contributes a deeper, richer, and more traditionally "musky" warmth, whereas Exaltolide excels in delicate, transparent, and ethereal musk accords.
Ambrettolide (Macrocyclic):
Profile: A sweet, soft, powdery, fruity (ambrette seed, dried fruit), and slightly waxy aroma, occasionally described as having a faint metallic or nutty facet. Extremely elegant and natural.
Performance: Demonstrates very good diffusion and tenacity, contributing to the naturalness and smoothness of a fragrance.
Comparison to Muscone: Ambrettolide shares the macrocyclic elegance but leans more towards a subtle fruity-waxy profile with less overt animalicity than Muscone. It often provides a more "vegetal" or "seed" musk quality, distinct from Muscone's mammalian warmth.
Galaxolide (Polycyclic Musk):
Profile: A clean, sweet, abstract, powdery, and slightly woody-floral scent. Often perceived as a "laundry" or "clean cotton" musk.
Performance: Extremely powerful, with excellent tenacity and high diffusion, rendering it a workhorse in functional perfumery.
Comparison to Muscone: Galaxolide represents a more abstract and "clean" musk profile, lacking the complex animalic and fatty nuances characteristic of Muscone. While highly versatile and cost-effective, it does not offer the same depth or naturalistic warmth that Muscone provides in fine fragrances.
Nitro Musks (e.g., Musk Ketone, Musk Xylene - Historically Significant):
Profile: Powdery, sweet, often with a slightly camphoraceous or bitter nuance. Musk Ketone specifically possessed a rich, sweet, animalic character.
Performance: Highly tenacious.
Comparison to Muscone: While some nitro musks like Musk Ketone shared a rich animalic character with Muscone, they are largely restricted or prohibited due to concerns regarding phototoxicity, neurotoxicity, and environmental persistence (Publishers Panel, n.d.). Muscone offers a safer, more sustainable alternative with a comparable, if not superior, olfactory quality in fine fragrance applications.
Muscone distinguishes itself through its balanced fusion of animalic depth and soft sweetness, providing a truly multifaceted and highly valued "classic" musk character that is indispensable in high-end perfumery.
References
Agoratopia. (n.d.). Muscone (Musk) Note. Retrieved from https://www.agoratopia.com/notes/muscone-musk
Baeyer, A. (1885). Ueber Polyacetylenverbindungen. Berichte der Deutschen Chemischen Gesellschaft, 18(2), 2277–2281.
Behr, A., & Neff, B. (2013). Industrial organic chemistry (5th ed.). Wiley-VCH.
ChemicalBook. (n.d.). Muscone CAS#: 541-91-3. Retrieved from https://m.chemicalbook.com/ProductChemicalPropertiesCB1378768_EN.htm
Kraft, P., Popaj, K., & Brauchli, R. (2000). A Concise Access to the Musk Odorant (−)-( R)-Muscone via a Novel Cycloaddition/Ring-Enlargement Sequence. Angewandte Chemie International Edition, 39(20), 3698–3700. https://doi.org/10.1002/1521-3773(20001016)39:20<3698::AID-ANIE3698>3.0.CO;2-H
Publishers Panel. (n.d.). Synthetic musk odorants in cosmetic products. Retrieved from https://publisherspanel.com/api/files/view/2307509.pdf
Ruzicka, L. (1926). Über die Konstitution des Muscons. Helvetica Chimica Acta, 9(1), 715–725. https://doi.org/10.1002/hlca.19260090196
The Allure of Musk: Exploring Perfumery's Most Seductive and Misunderstood Fragrance. (n.d.). NYC.PH. Retrieved from https://nyc.ph/blogs/perfume/the-allure-of-musk-exploring-perfumerys-most-seductive-and-misunderstood-fragrance
The Perfumers Apprentice. (n.d.). Muscone (Firmenich) SDS-9756. Retrieved from https://shop.perfumersapprentice.com/msds/9756.pdf
Yan, S., Wang, Y., Zhang, Y., Zhao, Y., Feng, X., Hou, Y., Ma, L., & Liu, Q. (2024). Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation. International Journal of Molecular Sciences, 25(2), 1215. https://pmc.ncbi.nlm.nih.gov/articles/PMC12030149/





