Floral Butanal (Florhydral®) - Technical Ingredient Overview
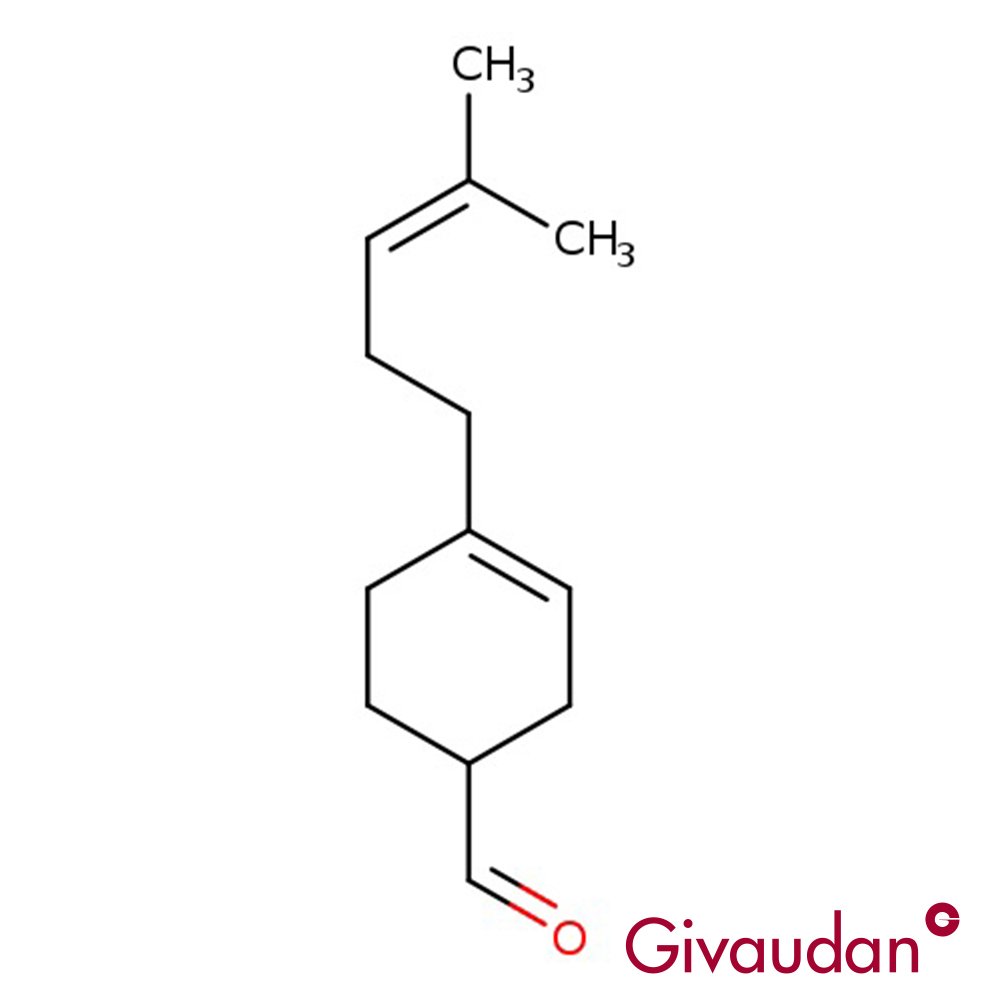
🔎 Chemical Name — 3-(3-Isopropylphenyl)butanal
🧪 Synonyms — Florhydral®, Floral Butanal, β-Methyl-3-(1-methylethyl)benzenepropanal, Isopropylphenylbutanal, 3-(3-propan-2-ylphenyl)butanal, Benzenepropanal, β-methyl-3-(1-methylethyl)-
📂 CAS Number — 125109-85-5
🆔 EINECS Number — 412-050-4
📘 FEMA Number — Not applicable (not approved for flavor use)
⚖️ Molecular Weight — 190.28 g/mol (190.29 g/mol)
📐 Molecular Formula — C₁₃H₁₈O
📝 Odor Type — Floral, Green, Aldehydic
📈 Odor Strength — Medium to Strong; highly diffusive
🌡️ Odor Threshold — 0.035 ng/L air [(+)-enantiomer]; 0.88 ng/L air [(-)-enantiomer]
👃🏼 Odor Profile — Fresh, muguet (lily of the valley), watery, green, aldehydic, slightly ozonic, with natural floral transparency. The racemate presents a balanced floral-green character with dewy freshness.
⚗️ Uses — Fine fragrance, functional perfumery (soaps, detergents, shampoos), personal care formulations, candles, burning applications
🧴 Appearance — Colorless to pale yellow liquid
🔬 Physical Properties —
Density: 0.935–0.954 g/cm³ at 25°C
Boiling Point: 251.3°C at 760 mmHg (257°C)
Flash Point: 103.6°C (68°C)
Vapor Pressure: 0.0200 hPa (0.65 Pa at 20°C)
Log P (octanol/water): 3.8
Refractive Index: 1.504–1.508 (n²⁰D)
What is Floral Butanal (Florhydral®)?
Floral Butanal, commercially known as Florhydral®, is a synthetic aliphatic aldehyde developed by Givaudan. It is classified chemically as a substituted benzenepropanal with a bulky isopropyl substituent at the meta-position of the phenyl ring. The molecule features an asymmetric carbon center, resulting in two enantiomeric forms with distinct olfactory properties (Bovo et al., 2008).
Florhydral® belongs to the aldehydic-floral chemical family and serves as a key building block for modern muguet (lily of the valley) reconstructions. Unlike many floral aldehydes, Florhydral® offers a naturalistic green nuance combined with high diffusivity and stability across diverse formulation matrices. The material exists as a racemic mixture in commercial use, though individual enantiomers have been synthesized for research purposes.
Chemical Classification:
Aromatic aldehyde
Substituted benzenepropanal
Aliphatic aldehyde with aromatic substituent
Positional isomer of Cyclamen Aldehyde
The compound does not occur naturally and is produced exclusively through synthetic routes (Chalk, 1988).
Historical Background
Florhydral® was developed by Givaudan during the late 20th century, emerging from the perfumery industry's growing demand for stable, allergen-reduced floral notes. The compound was first disclosed in US Patent 4,910,346, filed by A. Chalk for Givaudan Corporation and published on November 10, 1988 (Chalk, 1988).
Historical Context: The development of Florhydral® addressed a critical challenge in perfumery: the impracticality of natural muguet (lily of the valley) extraction. Convallaria majalis (lily of the valley) produces insufficient quantities of extractable volatile compounds for commercial perfumery, driving innovation toward synthetic interpretations throughout the 20th century. Earlier materials like hydroxycitronellal and Bourgeonal (p-tert-butyl-α-methylhydrocinnamaldehyde) dominated muguet compositions, but regulatory pressures and allergenicity concerns created demand for alternatives (Bauer et al., 2001).
Key Milestones:
1988: Patent filing and publication describing hydroformylation synthesis route
Late 1980s–1990s: Commercial introduction by Givaudan
2000s: Increased adoption following IFRA restrictions on competing materials
2006: Publication of improved synthetic methodologies including enantioselective routes (Scrivanti et al., 2006)
2010s–Present: Establishment as industry benchmark for modern muguet reconstructions, particularly following the EU ban on Lyral (2019–2021)
Florhydral® represents Givaudan's strategic response to combine fresh floral aldehydic impact with improved solubility, stability, and reduced allergen risk compared to earlier-generation materials (Sell, 2019).
Olfactory Profile
Scent Family
Primary: Floral-Green
Secondary: Aldehydic, Aquatic-Ozonic
Main Descriptors
Fresh: Clean, transparent, dewy character
Muguet: Classic lily of the valley signature
Green: Natural, vegetal undertones
Aldehydic: Crisp, airy quality typical of modern aldehydes
Watery: Aqueous, slightly marine aspect
Ozonic: Subtle airy freshness (less pronounced than dedicated ozonic materials)
The racemate delivers a balanced floral profile. The (+)-(S)-enantiomer exhibits a more pronounced green, less watery character with greater power (odor threshold 0.035 ng/L air). The (−)-(R)-enantiomer presents a more marine, plastic undertone with reduced intensity (odor threshold 0.88 ng/L air) (Bovo et al., 2008).
Intensity
Medium to strong diffusive strength. Florhydral® demonstrates remarkable intensity relative to many classical floral materials, making it effective at low concentrations (typically 0.2–2% in concentrate). The material's high volatility and low odor threshold ensure immediate top-note presence despite being technically a heart note material.
Tenacity
Moderate longevity typical of mid-weight aldehydes. Approximate one-week persistence on smelling strips under standard conditions. Substantivity varies by application:
Fine fragrance (alcohol): 4–6 hours on skin
Soap/detergent: excellent retention through wash cycle with persistent residual odor
Candles/burning formats: very good performance; stable under heat
Volatility
Medium volatility — Florhydral® functions as a heart note in technical classification due to molecular weight (190.28) and vapor pressure (0.02 hPa). However, its low odor threshold and high diffusivity create significant top-note impact in actual compositions. The material bridges traditional top/heart note boundaries.
Evaporation behavior:
Initial burst: aldehydic-green freshness (first 10–30 minutes)
Heart development: floral muguet character emerges (30 minutes–4 hours)
Dry-down: subtle watery-green traces (4–6 hours)
Fixative Role
Not a fixative. Florhydral® contributes to fragrance structure through its bridging volatility but does not extend the longevity of base notes. The material enhances radiance and lift in mid-phase accords, creating volume in floral bouquets without weighing down compositions.
Applications in Fine Fragrance
Florhydral® serves multiple strategic functions in modern perfumery:
Primary Applications
Muguet reconstructions: Core component for lily of the valley accords, often combined with Hydroxycitronellal (where permitted), Lilial alternatives, Cyclamen Aldehyde, and floral aldehydes
White floral bouquets: Adds transparent freshness to gardenia, jasmine, and tuberose compositions
Green floral accords: Enhances natural "stem" character in rose, hyacinth, and linden blossom
Citrus modifiers: Provides natural volume and floral lift to citrus top notes without heaviness
Aquatic florals: Bridges floral and ozonic families in marine-inspired fragrances
Typical Accords & Combinations
Classic Muguet Accord (example ratios):
Florhydral®: 20–30%
Hydroxycitronellal: 30–40% (where permitted)
Cyclamen Aldehyde: 10–15%
Helional: 5–10%
Aldehyde C-14 (Gamma Undecalactone): 3–5%
Geraniol/Linalool: 10–15%
Synergistic Pairings:
With aldehydes (Decanal, Undecanal): Creates "aldehydic note" signature; Florhydral® naturalizes synthetic aldehydic accords
With ozonic materials (Ultrazur®, Calone): Recommended 4:1 ratio (Florhydral®:Ultrazur®) produces aquatic-floral transparency
With citrus notes (Bergamot, Lemon, Grapefruit): Exalts citrus character while adding floral depth
With green notes (Cis-3-Hexenol, Galbanum, Violet Leaf): Reinforces natural stem/leaf aspects
Pairing Behavior
Florhydral® demonstrates excellent compatibility across functional groups. The material's neutral pH profile and chemical stability allow blending with:
Acid-sensitive materials (most perfume bases)
Oxidation-prone naturals (citrus oils)
Reactive aldehydes and ketones
Avoid in: Highly acidic bases (certain detergent formulations) and strong alkaline media (liquid bleach), where hydrolysis may occur.
Performance in Formula
Behavior in Blends
Florhydral® exhibits cooperative enhancement rather than dominance in mixtures. Key performance characteristics:
Diffusivity: High radiance creates immediate presence in headspace without overwhelming
Blending: Integrates seamlessly into both natural and synthetic frameworks
Stability: Resistant to oxidation; maintains character over typical product shelf life
Dosage response: Linear impact from 0.2% (subtle lift) to 2% (pronounced floral character); above 2%, aldehydic aspect may become dominant
Impact on Overall Composition
Volume and lift: Adds three-dimensional fullness to flat floral accords
Freshness modifier: Neutralizes heavy, indolic, or animalic undertones
Transparency: Creates "air" and space within dense compositions
Naturalization: Reduces synthetic perception in technical formulations
Stability Considerations:
Excellent in alcoholic solutions (perfumes, eaux de toilette)
Very good in emulsions (creams, lotions)
Good in anhydrous systems (oils, waxes)
Suitable for burning applications (candles, incense) — maintains character under heat
Compatible with soap matrices; survives saponification process
Recommended usage levels:
Fine fragrance: 0.2–2% of concentrate
Functional perfumery: 0.5–1% of concentrate
Muguet-focused compositions: up to 5–7% of concentrate
Soap perfumes: 1–2% of concentrate
Final Notes
Florhydral® (CAS 125109-85-5) represents a modern benchmark in synthetic floral perfumery. Its combination of naturalistic muguet character, regulatory freedom, and formulation versatility makes it an essential tool for contemporary perfumers. Following the phase-out of Lyral and increasing restrictions on traditional muguet materials, Florhydral® has assumed greater strategic importance in floral perfumery.
The material's lack of IFRA restrictions, absence from EU allergen lists, and excellent performance across fine and functional applications position it as a cornerstone ingredient for lily of the valley reconstructions and fresh floral compositions. Perfumers value Florhydral® for its ability to create natural transparency and volume in floral accords while maintaining technical stability and safety compliance.
Scentspiracy Recommendation: Florhydral® is suitable for perfumers at all skill levels. Begin testing at 0.5–1% in simple floral bases to understand its character before incorporating into complex compositions. The material's high diffusivity means small adjustments significantly impact headspace presence.
Industrial & Technical Uses
While Florhydral® is primarily a perfumery material, its applications extend to functional products:
Functional Perfumery Applications
Laundry products: Detergents, fabric softeners — valued for fresh residual odor on fabrics
Personal care: Shampoos, shower gels, body lotions — provides clean floral signature
Household products: Air fresheners, surface cleaners — contributes long-lasting freshness
Candles and incense: Excellent performance in burning formats; heat-stable
Technical Specifications for Formulation
Solubility: Soluble in ethanol, propylene glycol, diethyl phthalate (DEP), isopropyl myristate (IPM); limited solubility in water
pH stability: Stable in pH range 4–9; avoid extremes
Oxidative stability: Good resistance to oxidation; no antioxidant required for standard applications
Photostability: Moderate — recommend UV-protective packaging for long-term storage
Non-Fragrance Applications
Not applicable — Florhydral® has no approved food/flavor use (no FEMA GRAS status) and is not employed in pharmaceutical or industrial chemical synthesis outside perfumery.
Regulatory & Safety Overview
IFRA Status
Florhydral® (CAS 125109-85-5) is not restricted by IFRA as of Amendment 51 (current as of 2025). The material does not appear on the IFRA Standards or Prohibited Lists. Perfumers may use Florhydral® without category-specific limitations, making it a valuable alternative to restricted materials like Lyral (banned) and Bourgeonal (restricted to 0.5%) (IFRA, 2025).
IFRA Transparency List: Florhydral® appears on the IFRA Transparency List, requiring declaration when used in fragrance compounds for transparency purposes.
Note: While not restricted by IFRA, users should always consult the latest IFRA Amendment for updates. No official IFRA limit documentation available at IFRA official website.
EU Cosmetics Regulation
Permitted without specific restrictions under Regulation (EC) No 1223/2009. Florhydral® is not listed among the EU's 26 declarable fragrance allergens (Annex III), meaning it does not require individual declaration on cosmetic product labels (European Commission, 2009).
Standard safety assessments per Article 10 of the Cosmetics Regulation apply, requiring manufacturers to ensure safety at intended use levels.
REACH Status
Florhydral® is fully compliant with REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulations. The substance is registered under REACH with EINECS number 412-050-4 and is classified with the following harmonized classification:
GHS/CLP Classification:
H411: Toxic to aquatic life with long-lasting effects (Category 2)
Signal Word: Warning
Labelling Requirements:
Pictogram: GHS09 (Environment)
Hazard statement: H411
Precautionary statements: P273 (Avoid release to the environment), P391 (Collect spillage), P501 (Dispose according to regulations)
Allergen Risk
Low allergen potential. Florhydral® is not listed among the 26 fragrance allergens regulated under EU Cosmetics Regulation Annex III. The material is not classified as a skin sensitizer under CLP/GHS at normal usage concentrations (European Chemicals Agency, 2025).
Studies indicate low acute toxicity and absence of sensitization at recommended use levels (Firmenich, 2020). However, as with all fragrance materials, patch testing is recommended for sensitive individuals.
Toxicology
Acute Toxicity: Low; LD₅₀ values indicate minimal acute toxicity risk
Skin Sensitization: Not classified as a sensitizer at typical use concentrations
Reproductive Toxicity: No evidence of reproductive or developmental toxicity
Mutagenicity: No mutagenic activity detected in standard assays
Environmental Fate: Moderate aquatic toxicity (H411); biodegradable with proper treatment
Safety Profile Summary: Florhydral® demonstrates a favorable safety profile for cosmetic and perfumery applications when used according to good manufacturing practices and recommended dosage guidelines (Givaudan, 2020; Firmenich, 2020).
Additional Regulatory Notes
FDA Status (US): Not regulated as a cosmetic ingredient; generally recognized as safe for use in fragrances
California Prop 65: Not listed
Nordic Swan Ecolabel: Permitted; complies with general requirements for fragrances in cosmetics (O7, O9)
Synthesis & Chemical Considerations
Industrial Synthesis
Florhydral® is produced commercially via rhodium-catalyzed hydroformylation of m-diisopropenylbenzene (1,3-diisopropenylbenzene). This reaction introduces a formyl group (-CHO) to the double bond, yielding the aldehyde structure (Chalk, 1988; Scrivanti et al., 2006).
Synthesis Route:
Substrate: m-Diisopropenylbenzene
Catalyst: Rhodium complex (RhH(CO)(PPh₃)₃ or similar)
Conditions: 140°C, 17 atm syngas (CO/H₂ = 1:1)
Product: 3-(3-isopropenylphenyl)butyraldehyde (intermediate)
Hydrogenation: Catalytic reduction of remaining double bond yields Florhydral®
Key Challenges:
Conversion must be limited to ~40% to minimize formation of unwanted dialdehyde byproduct
Regioselectivity control critical for product purity
Alternative routes using 1-isopropyl-3-isopropenylbenzene provide better chemo- and regioselectivity
Enantioselective Synthesis: Academic research has demonstrated asymmetric hydroformylation and hydrogenation routes to enantiomerically enriched Florhydral® using chiral phosphine ligands (e.g., BINAP, Xantphos), achieving up to 97% enantiomeric excess (Bovo et al., 2008). However, commercial production uses the racemic mixture.
Chemical Stability
Oxidation: Resistant to autoxidation under normal storage conditions
Hydrolysis: Stable at neutral pH; susceptible to hydrolysis in strong acid or base
Polymerization: No polymerization tendency
Photolysis: Moderate photostability; store in amber glass or UV-protective containers
Storage & Handling
Storage Conditions:
Store in tightly sealed containers
Keep cool (15–25°C), dry, dark environment
Protect from light (amber glass recommended)
Keep away from oxidizing agents and strong acids/bases
Store under inert atmosphere (nitrogen) for long-term stability
Shelf Life: Typically 12–24 months under proper storage conditions
Handling Precautions:
Use in well-ventilated areas
Avoid release to environment (H411)
Wear appropriate PPE (gloves, eye protection) when handling neat material
Follow standard perfumery laboratory practices
References
Bauer, K., Garbe, D., & Surburg, H. (2001). Common fragrance and flavor materials: Preparation, properties and uses(4th ed.). Wiley-VCH. https://doi.org/10.1002/3527600213
Bovo, S., Scrivanti, A., Bertoldini, M., Beghetto, V., & Matteoli, U. (2008). A new enantioselective catalytic route to Florhydral®. Synthesis, 16, 2547–2550.
Chalk, A. (1988). Process for preparing aldehydes. US Patent 4,910,346. Givaudan Corporation.
European Chemicals Agency. (2025). Registered substances database. Retrieved from https://echa.europa.eu/information-on-chemicals/registered-substances
European Commission. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Official Journal of the European Union, L342/59.
Firmenich. (2020). Florhydral® technical data sheet. Internal technical documentation.
Givaudan. (2020). Florhydral™ product information. Retrieved from https://www.givaudan.com/fragrance-beauty/eindex/florhydraltm
IFRA. (2025). IFRA Standards: Amendment 51. International Fragrance Association. Retrieved from https://ifrafragrance.org/standards
PubChem. (2025). Isopropylphenylbutanal (CID 86209). National Center for Biotechnology Information. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/86209
Scrivanti, A., Bertoldini, M., Beghetto, V., Matteoli, U., & Venzo, A. (2006). Hydroformylation of m-diisopropenylbenzene and 1-isopropyl-3-isopropenylbenzene for the preparation of the fragrance Florhydral®. Journal of Molecular Catalysis A: Chemical, 247(1–2), 238–244. https://doi.org/10.1016/j.molcata.2005.11.047
Sell, C. (2019). Chemistry and the sense of smell. Wiley. https://doi.org/10.1002/9781118522981