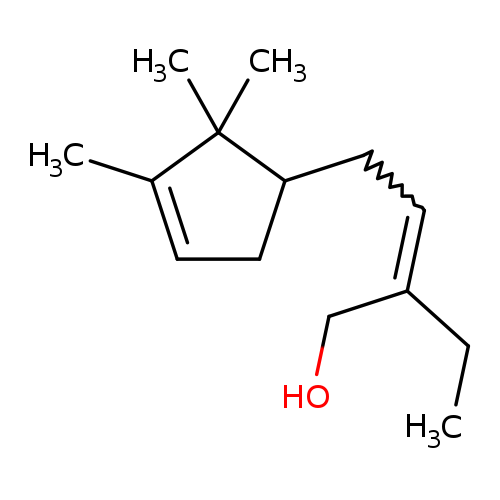
Polysantol® (107898-54-4) Technical Ingredient Overview
🏭 Manufacturer — Firmenich
🔎 Chemical Name — 3,3-Dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-penten-2-ol
🧪 Synonyms — Polysantol®, Santol Pentenol, Mysantol®, Tertiary Sandalwood Alcohol
📂 CAS Number — 107898-54-4
📘 FEMA Number — Not assigned
⚖️ Molecular Weight — 222.37 g/mol
🧬 Chemical Formula — C₁₅H₂₆O
📝 Odor Type — Woody (Sandalwood)
📈 Odor Strength — High intensity with exceptional diffusivity
👃🏼 Odor Profile — Powerful, creamy, diffusive sandalwood with smooth musky undertones, lactonic warmth, and subtle cedarwood aspects
⚗️ Uses — High-fidelity synthetic sandalwood substitute for woody, oriental, amber, and creamy floral compositions; functions as fixative and structure-builder
🧴 Appearance — Clear, colorless liquid
What is Polysantol®?
Polysantol® is a high-performance synthetic aroma chemical engineered to reproduce the olfactory signature of natural sandalwood (Santalum album). Developed and trademarked by Firmenich, this molecule represents a significant advancement in sustainable sandalwood alternatives, offering perfumers a cost-effective solution that maintains the complexity and sophistication of natural sandalwood without contributing to the depletion of endangered sandalwood trees.
The compound's chemical structure features a saturated aliphatic tertiary alcohol with a substituted cyclopentene ring, which accounts for its low volatility and exceptional substantivity (Surburg & Panten, 2006). Unlike earlier synthetic sandalwood materials that performed primarily in base notes, Polysantol® demonstrates remarkable versatility across all fragrance phases—top, heart, and base—making it uniquely valuable for modern perfumery (Pybus & Sell, 1999).
Polysantol® exhibits minimal batch-to-batch variation and demonstrates excellent stability in both alcohol-based fine fragrances and functional fragrance applications, including soaps, detergents, and personal care products.
Historical Background
The development of synthetic sandalwood substitutes intensified following India's 1977 export ban on Santalum albumoil, which created urgent demand for high-quality alternatives to this precious natural material. Early synthetic sandalwood molecules included Sandalore® (1976) and compounds based on isocamphylcyclohexanol chemistry.
Polysantol® was developed by Firmenich in the early 1980s as part of their research program on campholenic aldehyde-derived sandalwood chemicals. The compound emerged from systematic investigations into aldol condensation reactions between campholenaldehyde and various ketones, followed by selective reduction. The Polysantol® trademark was officially published and protected by Firmenich SA on October 22, 1985 (brand N°497404).
The molecule gained significant recognition following its prominent use in Guerlain's Samsara (1989), where it was employed at approximately 9.5% in the fragrance concentrate—an unusually high concentration that demonstrated the material's capacity to create powerful sandalwood effects. More recently, Polysantol® has been identified as the primary sandalwood component in Creed's Original Santal, used at approximately 0.85% in the finished fragrance, showcasing its efficiency at lower concentrations.
The compound's success catalyzed further research into campholenic aldehyde-derived sandalwood chemicals, leading to the development of related materials such as Javanol® (Givaudan, 2000) and contributing to a family of synthetic sandalwoods that have largely displaced natural sandalwood in commercial perfumery.
Olfactory Profile
Scent Family: Woody-Oriental / Sandalwood
Main Descriptors:
Top notes: Diffusive opening with creamy, lactonic character
Heart notes: Rich, smooth sandalwood with milky warmth and subtle musky facets
Base notes: Persistent woody-sandalwood foundation with gentle cedarwood nuances
Intensity: High odor value with exceptional diffusivity (>2×10⁵), significantly more powerful than natural sandalwood oil
Tenacity: Outstanding longevity, lasting over 336-400 hours on smelling strips; maintains olfactory presence for up to 2 weeks on fabric
Volatility: Low volatility due to tertiary alcohol structure; functions across all fragrance phases despite being classified as a base note material. The compound's unique diffusivity allows it to influence top and heart notes while anchoring the base
Fixative Role: Excellent fixative properties that enhance the longevity of volatile floral and citrus notes while adding substantial depth to the overall composition
Related Materials
Explore complementary synthetic sandalwood molecules and woody materials:
Javanol® - Constitutional isomer with similar structure, less milky character, higher power
Ebanol (Matsunol) - Four times stronger than natural sandalwood
Brahmanol® - Woody, tenacious sandalwood with slight musk nuance
Bacdanol® - Powerful sandalwood with slight rose nuance
Applications in Fine Fragrance
Polysantol® serves multiple essential functions in modern perfumery:
Primary Applications:
Sandalwood accords: Functions as primary sandalwood component or natural sandalwood modifier
Oriental and gourmand perfumes: Provides warm, creamy woody foundation
Modern woody bases: Essential structure-builder in contemporary woody-amber compositions
Creamy florals: Enhances jasmine, tuberose, frangipani, and gardenia with lactonic depth
Milky musks: Contributes smooth, skin-like warmth to musk compositions
Notable Pairings:
Demonstrates strong synergistic behavior with Iso E Super®, Javanol®, Brahmanol®, and Ambrettolide
Complements other sandalwood molecules (Sandalore®, Ebanol®, Bacdanol®) to create fuller, more complex sandalwood effects
Works beautifully with creamy lactones, vanilla, tonka bean, and amber materials
Harmonizes with modern woody-ambers and transparent musks
Typical Usage Levels:
Fine fragrance: 0.5-3% in perfume compound (limited by IFRA restrictions in finished product)
Functional fragrances: Higher concentrations possible due to excellent stability and bloom in surfactant systems
Historical Fragrance Examples:
Samsara by Guerlain (1989): 9.5% overdose showcasing Polysantol's® power
Original Santal by Creed: 0.85% in finished fragrance as primary sandalwood note
Performance in Formula
Polysantol® exhibits exceptional formulation characteristics. It is readily soluble in ethanol and other common perfumery solvents, demonstrating lipophilic behavior that ensures excellent incorporation into both alcohol and oil-based systems.
The compound shows good oxidative stability and maintains olfactory clarity even in challenging formulation environments, including high-surfactant systems such as shampoos, shower gels, and laundry products. Its stability in emulsions makes it particularly valuable for lotions and creams.
Performance characteristics include:
Boiling point: Approximately 290°C
Flash point: Greater than 100°C
Diffusion: Pronounced bloom in soaps and shampoos with excellent burning performance in candles
Stability: Less sensitive to pH changes than many naturals; maintains clarity in alkaline conditions
Industrial & Technical Uses
Beyond fine fragrance, Polysantol® finds extensive application in:
Cosmetics and personal care: Creams, lotions, body care products, and hair care formulations (0.1-10%)
Soaps and detergents: Excellent stability and bloom characteristics
Functional perfumery: Industrial fragrances, air care, and home care applications
Candles: Outstanding burning performance with consistent scent throw
Regulatory & Safety Overview
IFRA Status:
Restricted material under IFRA Amendment 51 (notified June 30, 2023)
Category 1 (Lip products): Maximum 0.031% in finished product
Category 4 (Fine fragrance): Maximum 1.1% in finished product (some sources cite up to 4%)
Full IFRA 51st Amendment Index available at: https://ifrafragrance.org/docs/default-source/51st-amendment/ifra-51st-amendment---index-of-ifra-standards.pdf
EU Cosmetics Regulation 1223/2009:
Compliant for use in cosmetic products
Not listed among the 26 declarable allergens
No specific skin sensitization classification
ECHA Classification:
H411: Toxic to aquatic life with long-lasting effects
Not classified as a skin sensitizer or irritant
REACH registered: Compliant with EU substance regulations
Environmental Considerations:
Biodegradability: Not readily biodegradable
Aquatic toxicity: H411 classification requires careful environmental stewardship
Sustainability advantage: High odor value allows effective use at low concentrations, offsetting material volume concerns
Safety Profile:
Generally well-tolerated in dermal applications
No evidence of sensitization potential in human repeated insult patch tests
Must be formulated with care to respect IFRA concentration limits and minimize environmental release
Storage & Handling:
Store in tightly sealed containers away from light and heat
Shelf life: ≥24 months under standard storage conditions
Stable under normal use conditions
Sustainability & Environmental Impact
Origin: Fully synthetic, produced via chemical synthesis from campholenaldehyde
Ecological Benefit: Polysantol® plays a crucial role in reducing demand for natural Santalum album, which is ecologically sensitive and increasingly protected by international trade laws (CITES Appendix II listing). The widespread adoption of high-quality synthetic alternatives like Polysantol® has significantly reduced pressure on wild sandalwood populations.
Environmental Considerations: While not readily biodegradable and classified as toxic to aquatic life (H411), the compound's exceptional odor value means effective results can be achieved at relatively low concentrations, partially offsetting environmental concerns associated with its use.
Batch Consistency: Unlike natural sandalwood oil, which varies significantly in quality and composition depending on geographic origin, age of trees, and harvest methods, Polysantol® offers consistent batch-to-batch performance, reducing formulation waste and reformulation needs.
References
Firmenich. (1985). Polysantol® trademark registration (Brand N°497404). Swiss Federal Institute of Intellectual Property.
International Fragrance Association. (2023). IFRA Standards: 51st Amendment [Index of Standards]. Retrieved from https://ifrafragrance.org/docs/default-source/51st-amendment/ifra-51st-amendment---index-of-ifra-standards.pdf
Pybus, D. H., & Sell, C. S. (Eds.). (1999). The chemistry of fragrances: From perfumer to consumer. Cambridge: Royal Society of Chemistry.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Weinheim: Wiley-VCH. https://doi.org/10.1002/3527608214