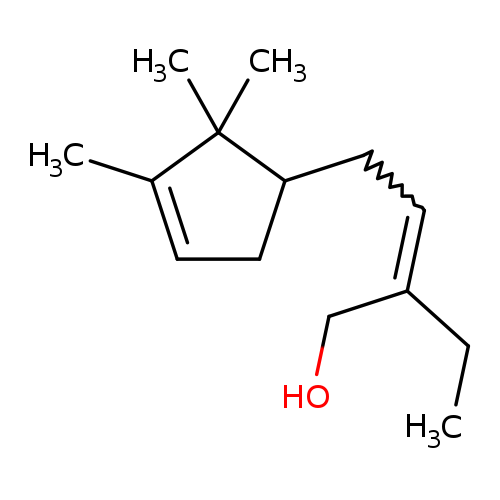
Technical Ingredient Overview
🏭 Manufacturer — Givaudan; DSM-Firmenich (marketed as Matsunol™)
🔎 Chemical Name — 3-Methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-penten-2-ol (CAS Common Chemistry, n.d.)
🧪 Synonyms — Ebanol®, Matsunol™, Mohanol, Muscosandrol, Sandal Pentenol, (E/Z)-diastereomeric mixture, 4-Penten-2-ol, 3-methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)- (CAS Common Chemistry, n.d.; Sigma-Aldrich, n.d.)
📂 CAS Number — 67801-20-1 (CAS Common Chemistry, n.d.)
📘 FEMA Number — 4775 (Givaudan, n.d.; Chemtex USA, n.d.)
⚖️ Molecular Weight — 208.34 g/mol (CAS Common Chemistry, n.d.)
📝 Odor Type — Woody-sandalwood, aromatic
📈 Odor Strength — Very high (approximately four times stronger than natural sandalwood oil); odor value exceeds 2 × 10⁵
👃🏼 Odor Profile — Rich, creamy sandalwood with pronounced ambery-musky facets; subtly lactonic and nutty in the dry-down; powerful and diffusive with aromatic nuances reminiscent of dried fennel, liquorice, and immortelle (Givaudan, n.d.)
⚗️ Uses — Fine fragrance compositions (woody and oriental accords), functional perfumery (soaps, shampoos, detergents), candle fragrances, fixative applications, powerful sandalwood oil replacer
🧴 Appearance — Colorless to pale yellow clear liquid (Givaudan, n.d.; BOC Sciences, n.d.)
What is Matsunol (Ebanol)?
Matsunol, more widely known by its Givaudan trade name Ebanol®, is a synthetic high-impact sandalwood odorant belonging to the trisubstituted cyclopentenol chemical class. The material represents a complex mixture of four diastereomeric secondary alcohols not found in nature, characterized by the presence of three asymmetric carbon centers and one carbon-carbon double bond that gives rise to both E- and Z-geometric isomers.
Structurally, Ebanol is classified as an α,β-unsaturated alcohol with a cyclopentene ring system, conferring exceptional tenacity and substantivity even at trace concentrations. The commercial material exhibits an extremely low odor threshold of approximately 0.21 ng/L in air, making it one of the most potent sandalwood molecules available to perfumers. Unlike natural sandalwood oil, which derives its characteristic odor primarily from α-santalol and β-santalol, Ebanol achieves a sandalwood impression through an entirely synthetic molecular architecture based on campholenic aldehyde chemistry.
The ingredient is valued in both fine fragrance and functional perfumery for its rich, natural sandalwood character combined with musky, leathery, and subtly fruity nuances. Its exceptional diffusion, substantivity across all supports, and cost-efficiency relative to natural sandalwood oil have established it as an indispensable tool in modern perfume formulation.
Historical Background
The development of Ebanol emerged from the fragrance industry's urgent need for sustainable sandalwood alternatives following the Indian government's 1977 export ban on Santalum album oil, which severely restricted access to one of perfumery's most precious raw materials (Government of India, 1977). This regulatory action, implemented to protect endangered sandalwood populations in Karnataka and other Indian states, catalyzed intensive research into synthetic sandalwood odorants throughout the late 1970s and early 1980s.
Early synthetic sandalwood milestones included Givaudan's Sandalore® (1976, European Patent EP 045453) and Firmenich's Polysantol® (1981, European Patent EP 115274), both representing significant advances in replicating natural sandalwood's olfactory profile. Building upon α-campholenic aldehyde chemistry pioneered in earlier research, Givaudan chemists Daniel Helmlinger and Mario Pesaro filed European Patent EP 116 277 with a priority date of January 13, 1983, covering the synthesis and stereochemical optimization of Ebanol® (Helmlinger & Pesaro, 1983).
The patent detailed a novel three-step synthesis involving aldol condensation of α-campholenaldehyde with 2-butanone, followed by base-catalyzed isomerization and selective reduction to yield the diastereomeric alcohol mixture. Industrial launch followed in 1986, with Ebanol rapidly gaining acceptance among perfumers for its exceptional odor value (exceeding 2 × 10⁵) and superior cost-efficiency compared to natural sandalwood oil. The molecule's success throughout the 1990s preceded the introduction of even more potent sandalwood synthetics such as Givaudan's Javanol® in 2000, yet Ebanol has retained its position as a workhorse ingredient due to its unique aromatic character and versatility across fragrance applications.
Olfactory Profile
Scent Family: Woody-sandalwood, aromatic
Main Descriptors: Ebanol delivers a rich, creamy sandalwood impression distinguished by pronounced ambery-musky facets and aromatic nuances. Unlike the more linear profiles of related sandalwood synthetics such as Sandalore®, Ebanol exhibits a complex olfactory character that perfumers describe as "aromatic-sandalwood," featuring sweet, woody notes reminiscent of dried fennel, liquorice, and immortelle (helichrysum) absolute (Perfume Chemicals, 2016). The material possesses a subtly lactonic quality that emerges in the dry-down, accompanied by nutty undertones that add warmth and depth. The musky aspect is particularly pronounced, differentiating Ebanol from more purely woody sandalwood synthetics and contributing to its exceptional fixative properties.
Intensity: Ebanol is classified as very high strength, with an odor threshold of approximately 0.21 ng/L in air—making it approximately four times more powerful than natural Santalum album oil on a weight-for-weight basis (Givaudan, n.d.). This exceptional potency allows effective use at concentrations as low as 0.1% in fragrance concentrates, though typical inclusion levels range from 0.5% for subtle volume enhancement to 3-5% for signature sandalwood cores in woody-oriental compositions.
Tenacity: Exceptional. Ebanol demonstrates remarkable substantivity, persisting for approximately 400 hours on blotter at 100% concentration, with Givaudan technical documentation recording detectable sandalwood character after three weeks of continuous evaluation (Givaudan, n.d.). This extraordinary tenacity significantly exceeds that of most natural sandalwood oils and positions Ebanol as a powerful fixative for more volatile fragrance components.
Volatility: Base note. As a relatively high-molecular-weight secondary alcohol (MW 208.34), Ebanol exhibits low volatility (vapor pressure 0.0013 hPa at 20°C) and functions primarily in the base accord of fragrance compositions (Givaudan, n.d.). While initial diffusion is pronounced due to the material's high odor value, the bulk of Ebanol's olfactory impact manifests in the heart-to-base transition and throughout the extended dry-down phase.
Fixative Role: Ebanol serves as a highly effective fixative, supporting volatile floral notes such as jasmine lactones and enhancing the longevity of lactonic gourmand accords. Its capacity to "round out and volumize" fruity and floral accords while providing long-lasting woody-musky support makes it particularly valuable in complex multi-faceted compositions where both olfactory character and technical performance are required.
Applications in Fine Fragrance
Ebanol occupies a versatile position in fine fragrance formulation, functioning both as a sandalwood oil replacer and as a multifunctional modifier that enhances depth, diffusion, and fixation across diverse olfactory families. Its pronounced musky-aromatic character distinguishes it from other sandalwood synthetics, making it particularly effective in compositions where a more complex, less purely "clean-woody" sandalwood impression is desired.
Role in Fragrance Compositions: Ebanol is widely employed as the sandalwood core in woody-oriental masculine fragrances and gourmand feminine compositions spanning the 1990s through contemporary perfumery. Industry sources indicate its presence in notable commercial fragrances including Dior Fahrenheit (post-2000 reformulations), Viktor & Rolf Flowerbomb, Givenchy Xeryus, Dolce & Gabbana Dolce Vita, Jean Paul Gaultier XS for Her, Clarins Elysium Pour Homme, Calvin Klein Escape for Men, Versace Red Jeans, and Chanel Égoïste, among others (Pell Wall Perfumes, n.d.). The material's exceptional cost-performance ratio has made it a staple in both prestige and mass-market fragrance formulation.
Typical Accords and Combinations: Ebanol demonstrates excellent compatibility with:
Woody accords: Enhances cedarwood, vetiver, and patchouli bases; blends synergistically with other sandalwood synthetics (Javanol®, Polysantol®, Bacdanol®) to create multidimensional sandalwood reconstructions
Oriental bases: Provides woody-musky foundation for amber, benzoin, vanilla, and labdanum compositions; particularly effective in supporting balsamic-resinous accords
Floral modifiers: Rounds out and volumizes rose, jasmine, and violet compositions while extending their tenacity
Fruity-gourmand structures: Adds depth and sophistication to fruit accords (particularly peach, plum, and berry notes); enhances lactonic-creamy gourmand effects
Pairing Behavior: The aromatic-musky character of Ebanol pairs exceptionally well with ionones, damascones, and rose materials, where it contributes a warm, enveloping base without overwhelming delicate floral nuances. In woody-spicy compositions, Ebanol harmonizes effectively with eugenol, cinnamon derivatives, and clove notes. Its subtle lactonic qualities make it a natural companion to coumarin, tonka, and vanillin in sweet-woody orientals.
Performance in Formula
Behavior in Blends and Mixtures: Ebanol exhibits excellent stability across a broad pH range (pH 2-11), maintaining its olfactory integrity in both acidic and alkaline formulations. The material is readily soluble in ethanol, dipropylene glycol (DPG), and other common perfumery solvents, though it remains immiscible with water—a typical characteristic of lipophilic fragrance alcohols.
Technical evaluations by Givaudan demonstrate superior diffusion performance in soap applications (rated 5/5), excellent burning effectiveness in candle wax, and high substantivity in both damp (wet) and dry (fabric/skin) conditions (Givaudan, n.d.). Unlike some sandalwood synthetics that can exhibit instability in strongly acidic detergent bases or chlorine-containing bleach formulations, Ebanol maintains reasonable stability, though prolonged exposure to extreme alkaline conditions (liquid bleach) may cause some olfactory degradation.
Impact on Overall Composition: Ebanol functions as a powerful volume-builder in fragrance compositions, contributing what perfumers describe as "elegance and diffusive sandalwood effect" without dominating more delicate top and heart notes (Givaudan, n.d.). At lower concentrations (0.1-0.5%), it provides subtle warmth and enhances the perceived quality of woody accords. At moderate levels (1-3%), it establishes a clear sandalwood signature while retaining transparency. At higher inclusion rates (3-5%), Ebanol can serve as the primary woody-musky base, though perfumers note that excessive use may accentuate the aromatic-anisic facets, potentially overwhelming more nuanced olfactory effects.
Storage and Handling: Technical literature recommends storing Ebanol in cool conditions within well-sealed containers to prevent oxidative degradation and off-odor development (Pell Wall Perfumes, n.d.). Dilution in ethanol or DPG shortly after opening is advisable for long-term stability, particularly in warm climates or facilities without temperature control.
Industrial & Technical Uses
Beyond fine fragrance applications, Ebanol serves diverse functions across the broader fragrance industry:
Functional Perfumery: The material's exceptional substantivity and stability make it highly effective in functional applications including fabric care products (laundry detergents, fabric softeners), personal care items (shampoos, shower gels, body lotions), and household cleaning formulations. Its performance in soap bases is particularly notable, with industry sources citing excellent diffusion and lasting power even through the saponification process and rinse-off conditions.
Candle Fragrance: Ebanol demonstrates excellent burning effectiveness in paraffin, soy, and blended wax systems, maintaining its olfactory profile throughout the candle's burn life (Givaudan, n.d.). The material's low volatility and high flash point (boiling point 283°C) contribute to predictable cold-throw and hot-throw performance.
Flavor Applications: With FEMA GRAS status (FEMA No. 4775), Ebanol is approved for use in regulated flavor concentrations, though its primary commercial application remains in fragrance (Givaudan, n.d.; Chemtex USA, n.d.). Flavor applications are limited due to its pronounced aromatic character, which does not closely replicate natural sandalwood's taste profile.
Analytical Standard: Pure Ebanol serves as an analytical reference standard for detection and quantification in sandalwood oil analysis, cosmetic product testing, and biological samples from animals exposed to sandalwood odorants, using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) techniques (Chemical Book, n.d.).
Regulatory & Safety Overview
IFRA Status: Ebanol is not subject to specific usage restrictions under IFRA Amendment 51 (notified June 30, 2023), allowing unrestricted use across all product categories provided general safety and formulation best practices are followed. The material does not appear in the IFRA Standards Library under prohibition, restriction, or specification categories through the 51st Amendment.
EU Cosmetics Regulation: Ebanol is classified as a fragrance allergen under European Regulation (EU) 2023/1545 (dated August 26, 2023), which amended Regulation (EC) No. 1223/2009 on cosmetic products (EUR-Lex, 2023). Under this regulation:
Mandatory labeling is required when Ebanol concentration exceeds 0.001% in leave-on cosmetic products
Mandatory labeling is required when Ebanol concentration exceeds 0.01% in rinse-off cosmetic products
Transition periods allow products formulated before July 31, 2026 to remain on the market until July 31, 2028 without updated labeling
This classification reflects the expanded EU allergen list, which increased from 24 to over 80 substances requiring declaration to enhance consumer transparency and safety (Cosmeservice, 2025).
FEMA Status: Ebanol holds FEMA GRAS (Generally Recognized As Safe) approval with designation FEMA No. 4775 for use in regulated flavor concentrations (Givaudan, n.d.; Chemtex USA, n.d.). This status permits limited flavor applications under IOFI (International Organization of the Flavor Industry) Code of Practice guidelines.
GHS Classification: Safety data sheets indicate the following hazard classifications:
H411: Toxic to aquatic life with long-lasting effects (Perfume Extract UK, n.d.)
Precautionary statements include P273 (Avoid release to the environment) and P501 (Dispose of contents/container in accordance with national regulations)
Toxicology: While comprehensive toxicological data are proprietary to manufacturers, publicly available safety assessments indicate that Ebanol has undergone standard RIFM (Research Institute for Fragrance Materials) evaluation processes typical for fragrance ingredients. The material's allergen classification under EU Regulation 2023/1545 reflects sensitization potential at higher concentrations, consistent with many fragrance alcohols. Professional use requires adherence to standard safety protocols including adequate ventilation, use of personal protective equipment during handling of concentrated material, and compliance with occupational exposure limits established by regulatory authorities.
Environmental Considerations: Givaudan's Eco-Compass™ scorecard assessment indicates that Ebanol is readily biodegradable and comprises greater than 50% renewable-carbon origin, contributing to improved sustainability profiles compared to some alternative synthetic sandalwood materials. The H411 aquatic toxicity classification necessitates responsible disposal practices and prevention of release into aquatic environments.
Sources:
BOC Sciences. (n.d.). CAS 67801-20-1 Ebanol. Retrieved from https://www.bocsci.com/product/ebanol-cas-67801-20-1-393337.html
CAS Common Chemistry. (n.d.). Ebanol (CAS RN: 67801-20-1). CAS, a division of the American Chemical Society. Retrieved from http://commonchemistry.cas.org/detail?cas_rn=67801-20-1
Chemical Book. (n.d.). Ebanol | 67801-20-1. Retrieved from https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8424233.htm
Chemtex USA. (n.d.). Ebanol. Product specifications. Retrieved from https://chemtex-prototype.squarespace.com/flavor-fragrance/ebanol
Cosmeservice. (2025, June 19). Regulation 2023/1545 changes the allergen list. Retrieved from https://cosmeservice.com/news/regulation2023-1545-changes-the-allergen-list/
EUR-Lex. (2023). Commission Regulation (EU) 2023/1545 of 26 July 2023 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards labelling of fragrance allergens in cosmetic products. Official Journal of the European Union, L 188. Retrieved from https://eur-lex.europa.eu/eli/reg/2023/1545/oj/eng
Givaudan. (n.d.). Ebanol™. Givaudan fragrance molecules. Retrieved from https://www.givaudan.com/fragrance-beauty/fragrance-ingredients-business/fragrance-molecules/ebanoltm
Government of India. (1977). Export ban on Santalum album oil.
Helmlinger, D., & Pesaro, M. (1983). Campholenic alcohol derivatives (European Patent No. EP 116 277). European Patent Office.
Pell Wall Perfumes. (n.d.). Ebanol. Product description and technical information. Retrieved from https://pellwall.com/products/ebanol
Perfume Chemicals. (2016, June 17). Sandalwood-like synthetics. Retrieved from https://perfumechemicals.wordpress.com/2016/06/17/sandalwood-like-synthetics/
Perfume Extract UK. (n.d.). Ebanol Givaudan 10. Product safety and technical data. Retrieved from https://perfumeextract.co.uk/product/ebanol-givaudan-10-dpg/
Sigma-Aldrich. (n.d.). CAS 67801-20-1. Product catalog information. Retrieved from https://www.sigmaaldrich.com/US/en/search/67801-20-1