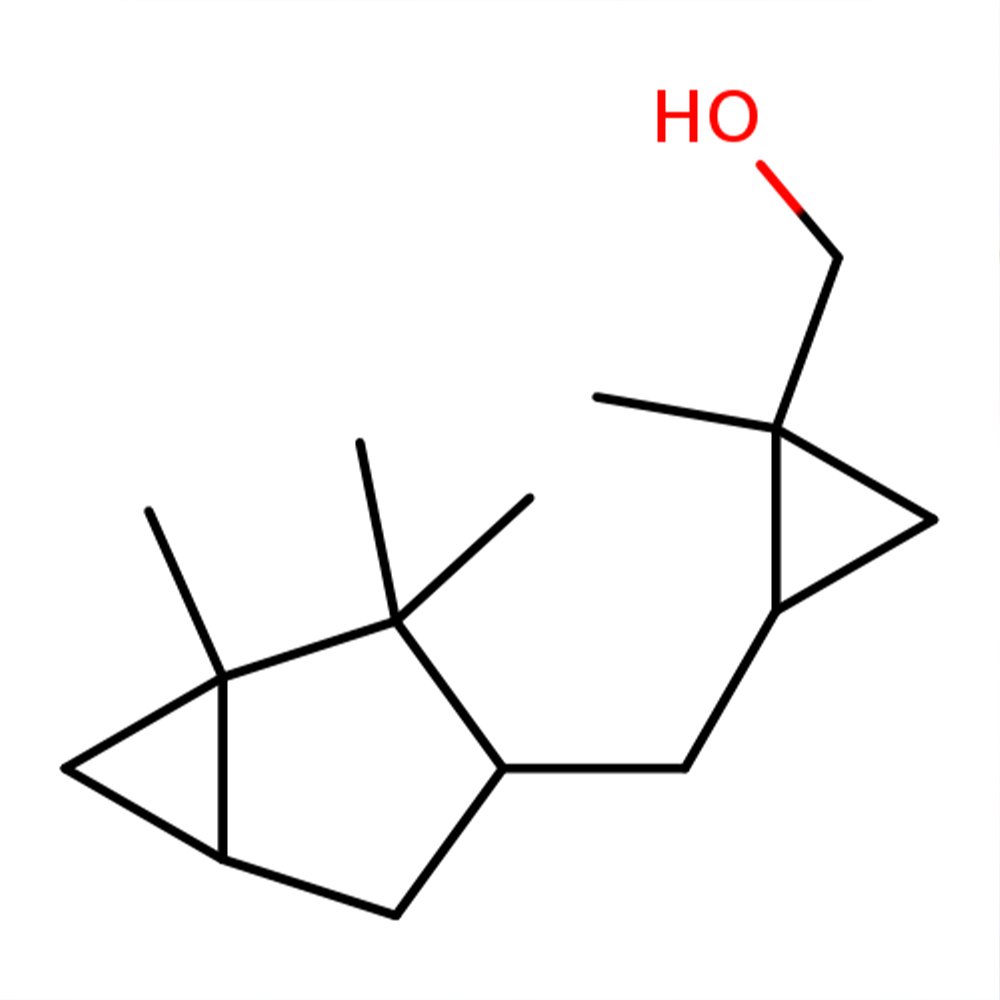
Technical Ingredient Overview
🏭 Manufacturer — Givaudan SA
🔎 Chemical Name — [1-Methyl-2-[(1,2,2-trimethylbicyclo[3.1.0]hex-3-yl)methyl]cyclopropyl]methanol (mixture of diastereoisomers)
🧪 Synonyms — Sandal cyclopropane; Java Sandalwood; Cyclopropanemethanol, 1-methyl-2-[(1,2,2-trimethylbicyclo[3.1.0]hex-3-yl)methyl]-
📂 CAS Number — 198404-98-7
📘 FEMA Number — 4776
📐 EC Number — 427-900-1
⚖️ Molecular Weight — 222.37 g/mol
📝 Molecular Formula — C₁₅H₂₆O
📈 Odor Type — Woody, Sandalwood
📊 Odor Strength — Exceptionally high; odor threshold of 0.02 ng/L air (20 ppt)
👃🏼 Odor Profile — Rich, creamy sandalwood with rosy-floral nuances; powerful, diffusive, and exceptionally tenacious with metallic-citric grapefruit facets and milky-lactonic undertones
⚗️ Uses — Fixative and sandalwood base for fine fragrance; modifier in woody, floral, musky, and amber accords; functional perfumery for soaps, detergents, and cosmetics
🧴 Appearance — Colorless to pale yellow viscous liquid
What is Javanol?
Javanol is a high-impact synthetic sandalwood odorant discovered in 1996 by chemist Jerzy Bajgrowicz at Givaudan (Escentric Molecules, 2017). This molecule represents a breakthrough in sandalwood chemistry, achieving what many consider the closest synthetic approximation to the olfactory receptor binding profile of natural β-santalol—the principal odor-active component in East Indian sandalwood oil (Santalum album) (Ohloff, Pickenhagen, & Kraft, 2012).
Javanol belongs to the campholenic aldehyde-derived sandalwood family and is characterized by multiple asymmetric carbon centers, resulting in a mixture of diastereomeric isomers used commercially in perfumery (Givaudan, 2024). Unlike its structural precursor, the molecule features two cyclopropane rings that replace reactive double bonds, creating a rigid, electron-rich framework optimized for olfactory receptor activation (Bajgrowicz, Frank, Frater, & Hennig, 1998).
The chemical is classified as a tertiary cyclopropyl alcohol within the bicyclic terpene-derived class of fragrance materials. Its molecular architecture combines hydrophobic bulk with precisely positioned hydrogen-bonding capability—a structural motif essential for sandalwood odor character (Fráter, Bajgrowicz, & Kraft, 1998).
Historical Background
Discovery and Development
Javanol was discovered in 1996 by Jerzy Bajgrowicz, working in collaboration with Georg Frater at Givaudan's fragrance research center in Dübendorf, Switzerland (Escentric Molecules, 2017). The discovery emerged during Givaudan's intensive research program focused on macrocyclic and alicyclic fragrance molecules, particularly in response to the growing scarcity and escalating cost of natural sandalwood oil (Gaillard, 2007).
By the mid-1990s, the fragrance industry was engaged in what has been described as a "sandalwood arms race," with major houses including Givaudan, IFF, Firmenich, Kao, and Symrise all developing synthetic alternatives (Escentric Molecules, 2017). While Givaudan already possessed several successful sandalwood molecules—including Sandela, Radjanol, and Ebanol—and competitors had introduced Bacdanol (IFF), Polysantol (Firmenich), and Brahmanol (Symrise), none matched the receptor-binding efficiency ultimately achieved by Javanol (Escentric Molecules, 2017).
First Synthesis and Patent Protection
The synthesis of Javanol was first disclosed in U.S. Patent 5,707,961, filed in April 1996 and granted in January 1998 to Givaudan-Roure (International) SA, with inventors Jerzy A. Bajgrowicz and Georg Frater (Bajgrowicz & Frater, 1998). The patent covered novel odorants derived from campholenic aldehyde through double cyclopropanation methodology.
Year of Commercial Introduction
Javanol was officially introduced to perfumers in 1997 (Yudov, 2017), though its discovery and initial synthesis occurred in 1996. The molecule quickly gained recognition for its unprecedented odor threshold and unique olfactory characteristics, establishing itself as a benchmark material in modern sandalwood perfumery (Gautschi, Bajgrowicz, & Kraft, 2001).
Olfactory Profile
Scent Family
Primary Classification: Woody – Sandalwood
Secondary Classifications: Floral (rose facet), Citrus (grapefruit nuance), Musky (clean lactonic aspect)
Main Descriptors
Javanol presents a powerful, creamy sandalwood note reminiscent of β-santalol, combined with distinctive rosy nuances (Givaudan, 2024). The molecule exhibits a unique "almost psychedelic freshness" with what perfumer Geza Schoen describes as "liquid metallic grapefruit peel poured over a bed of velvety cream-colored roses" (Escentric Molecules, 2017).
The olfactory character includes:
Primary facets: Creamy sandalwood, milky-lactonic, woody warmth
Secondary facets: Rosy-floral brightness, citric grapefruit aspects, subtle musk
Tertiary facets: Dry vetiver undertones, metallic-cologne freshness, clean powdery nuances (Gaillard, 2007)
According to perfume chemist Arcadi Boix Camps, Javanol is unique among synthetic sandalwood molecules in that it captures both the aldehydic and alcoholic character of natural sandalwood oil, making it "perhaps the best of all" sandalwood synthetics and "impossible to replace" (cited in Pellwall, 2024).
Intensity
Javanol possesses an extraordinarily low odor threshold of 0.02 ppt (parts per trillion), or 0.02 nanograms per liter of air—making it approximately 400 times more potent than its closest analog, Pashminol (Yudov, 2017). This odor threshold represents one of the lowest values recorded for any sandalwood-type molecule (Ohloff, Pickenhagen, & Kraft, 2012).
In wash tests, Javanol demonstrates approximately 8 times greater effectiveness than the most powerful competing sandalwood products (Perfumer's Apprentice, 2024), attributed to its superior receptor binding affinity and resistance to volatilization.
Tenacity
Javanol exhibits exceptional substantivity, lasting over 400 hours on blotter—significantly exceeding the performance of natural sandalwood oil and competing synthetics. This extraordinary longevity derives from:
High molecular weight (222.37 g/mol)
Tertiary alcohol functionality with steric shielding
Extremely low vapor pressure (0.0003 hPa at 20°C) (Givaudan, 2024)
Strong lipophilic character (Log P = 4.8)
Volatility
Boiling Point: 268°C (at atmospheric pressure) (Givaudan, 2024)
Evaporation Rate: Very slow (base note category)
Volatility Classification: Heavy base note with exceptional fixative properties
Despite its base note classification, Javanol demonstrates unusual radiance and diffusivity, projecting effectively even at trace concentrations (Givaudan, 2024). This paradoxical behavior—combining low volatility with high perceptibility—results from its remarkably low odor threshold and optimal receptor binding.
Fixative Role
Because of its exceptional performance and stability, Javanol functions as the structural backbone for modern sandalwood notes and can enhance substantivity across all fragrance phases (Gaillard, 2007). Perfumer Antoine Gaillard notes that "a very small dosage of Javanol (0.02%) can produce a boosting effect, conferring additional volume and improved substantivity to more traditional sandalwood-type compositions" (Gaillard, 2007).
The molecule's fixative properties extend beyond sandalwood accords: Javanol's additional olfactive facets and low threshold enable trace amounts to impart volume and radiance effects to all types of perfumes (Gaillard, 2007), functioning as what industry professionals term a "radiant fixative."
Applications in Fine Fragrance
Role in Fragrance Compositions
Javanol serves as the backbone for all modern sandalwood notes due to its performance and stability (Gaillard, 2007). However, perfumers recognize that the very specific scent of sandalwood oil is difficult to reproduce with a single molecule, and consequently, a mixture of several synthetic sandalwood substitutes is necessary to obtain the complete olfactory profile of natural sandalwood oil (Gaillard, 2007).
Primary Applications:
Sandalwood Reconstruction: Core material in sandalwood bases and accords
Woody-Oriental Compositions: Provides creamy depth and warmth
Floral Modulation: Enhances rose, jasmine, and lily-of-the-valley with rosy-woody facets
Musky Accords: Contributes clean, lactonic warmth to modern musks
Amber Structures: Adds fixative strength and creamy-woody character
Typical Accords and Combinations
Javanol demonstrates notable synergy with Nectaryl, creating an unexpected "reminiscence of a pencil freshly carved at school" with surprising inflections of peach and patchouli (in a 20:80 ratio) (Perfumer's Apprentice, 2024).
Recommended Pairings:
With other sandalwood synthetics: Polysantol (for increased sweetness and milkiness), Ebanol (for musky-leathery depth), Bacdanol (for soft, powdery effects)
With natural materials: Vetiver oil, patchouli, rose absolute, jasmine, bergamot, labdanum, oakmoss
With synthetics: Iso E Super, Cashmeran, Hedione, ionones, musks, amber bases
Pairing Behavior with Other Ingredients
The molecule's strong sandalwood character can "saturate" specific olfactory receptors at low concentrations, subsequently activating less specific neurons that code for lactonic, rose, lily-of-the-valley, and grapefruit notes (Escentric Molecules, 2017). This combinatorial receptor activation explains Javanol's complex, multi-faceted character.
Formulation Considerations:
Can skew overly "rosy" if overdosed; careful modulation with lactones, ambers, or other woody materials is recommended
Many perfumers find Javanol so strong that it requires very judicious dosing (Pellwall, 2024)
Works exceptionally well in trace amounts (0.02–0.1%) as a radiance booster
Typical fine fragrance usage: 0.5–2% in compound
Notable Fragrances Featuring Javanol:
Truth for Men (Calvin Klein), Chic for Men (Carolina Herrera) (Pellwall, 2024)
Orto Parisi Bergamask (Alessandro Gualtieri) (Yudov, 2017)
Molecule 04 and Escentric 04 (Escentric Molecules, Geza Schoen)
Performance in Formula
Behavior in Blends and Mixtures
Javanol offers excellent oxidative and thermal stability, making it suitable for most applications except highly reactive systems like bleach. The molecule's stability profile includes:
Chemical Stability:
✅ UV-stable (not photolabile)
✅ Oxidation-resistant under normal conditions
✅ Thermally stable up to moderate temperatures
✅ Acid/base stable under mild pH conditions (pH 4–9)
❌ Not bleach-resistant (alcohol functionality vulnerable)
Physical Stability:
Excellent solubility in alcohol, dipropylene glycol, and fragrance oils
Maintains clarity in aqueous-alcoholic systems
Non-discoloring in most applications
Minimal reactivity with aldehydes, ketones, and esters
Impact on Overall Composition
Javanol's behavior during evaporation varies depending on the type of application in which it was incorporated (Gaillard, 2007), demonstrating context-dependent olfactory evolution. In alcohol-based fine fragrances, the molecule provides:
Opening Phase: Surprisingly radiant projection despite base note classification
Heart Development: Emergence of rosy-floral and citric facets
Drydown: Persistent creamy sandalwood with musky warmth
Technical Performance Metrics:
Diffusivity: High (despite low vapor pressure)
Bloom: Excellent radiance on skin and textiles
Wash Fastness: Superior retention through multiple launderings
Substantivity: Exceptional on cellulosic and protein fibers
Industrial & Technical Uses
Non-Fragrance Applications
Javanol is FEMA-approved (FEMA 4776) for use in flavoring applications (Givaudan, 2024), though it is not widely used in flavor work. The molecule's flavor profile includes woody and amber characteristics that may contribute to certain specialty flavor systems.
Industrial Processes and Functions
Functional Perfumery Applications:
Fabric care products (detergents, softeners, dryer sheets)
Personal care (shampoos, body washes, lotions, creams)
Household products (air fresheners, cleaning agents)
Candles and home fragrance systems
Manufacturing Considerations:
Synthesized via double Simmons-Smith cyclopropanation from 2-methyl-4-(2,2,3-trimethyl-3-cyclopenten-1-yl)-2-buten-1-ol, involving zinc and copper catalysis with diiodomethane (Givaudan, 2024)
Derived from naturally occurring α-pinene (obtained from pine turpentine) (Gaillard, 2007)
Commercial material is a mixture of diastereoisomers; Javanol® Super represents a single isolated isomer with enhanced performance (Givaudan, 2024)
Technical Specifications and Requirements
Storage Requirements:
Store in tightly closed containers
Keep away from heat, sparks, and open flames
Recommended storage temperature: 15–25°C
Shelf life: 24 months under proper storage conditions
Handling Precautions:
Classified as skin sensitizer (Category 1)
Use appropriate personal protective equipment
Avoid prolonged skin contact
Ensure adequate ventilation during formulation
Regulatory & Safety Overview
IFRA Status
Javanol does not contain any allergens listed among the 26 declarable fragrance allergens under EU Cosmetics Regulation (Givaudan, 2024). According to current IFRA Standards (through the 51st Amendment, notified June 30, 2023), Javanol is not restricted (International Fragrance Association, 2023).
IFRA Amendment 51 Status: ✅ Not restricted
Official IFRA Documentation: https://ifrafragrance.org/safe-use/library
However, general usage guidance suggests levels up to 2% in finished products (equivalent to 20% in fragrance concentrate) based on industry best practices and formulation performance optimization rather than regulatory limitation.
EU Cosmetics Regulation
Compliance Status: ✅ Compliant for use in cosmetic products
Allergen Declaration: Not required (not among the 26 declarable substances)
CLP Classification: Skin sensitizer Category 1
Under EU Regulation (EC) No 1223/2009 on cosmetic products, Javanol may be used in cosmetic formulations when properly declared on ingredient lists and subject to standard safety assessment requirements.
FEMA Status
FEMA Number: 4776 (Givaudan, 2024)
Classification: Approved for use as a flavoring substance
Usage: Limited application in flavor systems due to predominantly woody-aromatic profile
Toxicology
Based on industry safety data and RIFM assessments (Research Institute for Fragrance Materials):
Acute Toxicity: No significant concerns at normal usage levels
Skin Sensitization: Classified as Category 1 skin sensitizer; appropriate handling protocols required
Environmental Profile: No major concerns related to bioaccumulation or aquatic toxicity reported under normal usage conditions
Safety Recommendations:
Follow standard industry safety protocols during handling and formulation
Conduct appropriate skin sensitization testing in finished product formulations
Adhere to good manufacturing practices (GMP)
Maintain proper documentation of safety assessments
Sustainable Production and Environmental Considerations
Renewable Feedstock
Javanol is derived from α-pinene, a naturally occurring terpene obtained from pine turpentine (Gaillard, 2007), making it significantly more sustainable than natural sandalwood oil from endangered Santalum album trees. The starting material originates from forestry byproducts, representing a renewable and non-threatened resource.
Conservation Impact
The development of high-performance sandalwood synthetics like Javanol directly addresses the growing scarcity and escalating price of natural sandalwood oil, which has resulted from overharvesting of slow-growing Santalum species (Gaillard, 2007). East Indian sandalwood (Santalum album) has been listed on the IUCN Red List since 2004, and the Indian government has implemented protective legislation to prevent over-exploitation (Arctander, 1960).
By providing a viable alternative to natural sandalwood oil, Javanol contributes to conservation efforts while enabling perfumers to create sandalwood accords without depleting endangered tree populations.
Advantages and Limitations
Key Advantages
✅ Exceptional Potency: Lowest odor threshold among sandalwood synthetics (0.02 ng/L air)
✅ Unmatched Tenacity: Over 400 hours substantivity on blotter
✅ Superior Receptor Binding: Optimized electronic shape for sandalwood perception
✅ Excellent Stability: UV-stable, oxidation-resistant, thermally robust
✅ Multi-Functional: Serves as backbone, modifier, booster, and radiant fixative
✅ Renewable Source: Derived from pine-sourced α-pinene
✅ No IFRA Restrictions: Unrestricted use under current standards
✅ Non-Allergenic: Not among the 26 declarable allergens
Limitations and Considerations
⚠️ Overdose Sensitivity: Can skew overly "rosy" at high concentrations
⚠️ Not Bleach-Resistant: Alcohol functionality vulnerable in aggressive oxidative systems
⚠️ Skin Sensitization: Classified as Category 1 sensitizer; requires proper handling
⚠️ Single-Note Limitation: Cannot fully replicate the complexity of natural sandalwood oil as a standalone ingredient
⚠️ Cost Factor: Premium pricing reflects complex synthesis and exceptional performance
⚠️ Abstraction Character: Represents an "abstraction of santalness" rather than complete natural oil replication
Related Scentspiracy Database Entries
Structural Relatives
Polysantol® Polysantol® (107898-54-44-) — Constitutional isomer of Javanol with similar structure; exhibits a more pronounced milky note but lower potency (Givaudan, 2024)
Ebanol/Matsunol (67801-20-1) — Precursor in the campholenic aldehyde family; mixture of diastereomeric alcohols with musky-leathery facets (Surburg & Panten, 2016)
Bacdanol (28219-61-6) — IFF sandalwood synthetic with soft milky undertones and excellent stability (Surburg & Panten, 2016)
Brahmanol (72089-08-8) — Symrise sandalwood alcohol with 69% renewable carbon content and creamy-musky character (Symrise, 2022)
Natural Comparisons
Sandalwood Oil (8006-87-9) — Natural essential oil from Santalum album with complex woody-lactonic profile (Arctander, 1960)
Complementary Materials
Cedramber (67874-81-1) — Dry woody-amber from cedarwood chemistry; pairs well with sandalwood synthetics (Surburg & Panten, 2016)
Sources
Arctander, S. (1960). Perfume and Flavor Materials of Natural Origin. Published by the author.
Bajgrowicz, J. A., & Frater, G. (1998). Odorant compounds (U.S. Patent No. 5,707,961). United States Patent and Trademark Office.
Bajgrowicz, J. A., Frank, I., Frater, G., & Hennig, M. (1998). Synthesis and structure elucidation of a new potent sandalwood-oil substitute. Helvetica Chimica Acta, 81(8), 1349–1358. https://doi.org/10.1002/hlca.19980810802
Escentric Molecules. (2017). A masterclass on Molecule 04. Retrieved from https://www.escentric.com/en-us/blogs/news/a-masterclass-on-molecule-04
Fráter, G., Bajgrowicz, J. A., & Kraft, P. (1998). Fragrance chemistry. Tetrahedron, 54(24), 7633–7703. https://doi.org/10.1016/S0040-4020(98)00199-9
Gaillard, A. (2007, January). Perfumer's notes: Javanol. Perfumer & Flavorist, 32, 38–40.
Gautschi, M., Bajgrowicz, J. A., & Kraft, P. (2001). Fragrance chemistry—Milestones and perspectives. Chimia, 55(5), 379–387.
Givaudan. (2024). Javanol—Technical data sheet. Retrieved from https://www.givaudan.com/fragrance-beauty/fragrance-ingredients-business/fragrance-molecules/javanol
International Fragrance Association. (2023). 51st Amendment to the IFRA Standards. Retrieved from https://ifrafragrance.org/safe-use/library
Ohloff, G., Pickenhagen, W., & Kraft, P. (2012). Scent and Chemistry: The Molecular World of Odors. Verlag Helvetica Chimica Acta and Wiley-VCH.
Pellwall. (2024). Javanol—Product description. Retrieved from https://pellwall.com/products/javanol
Perfumer's Apprentice. (2024). Javanol® (Givaudan)—Product information. Retrieved from https://shop.perfumersapprentice.com/p-6135-javanol-givaudan.aspx
Pybus, D. H., & Sell, C. S. (Eds.). (1999). The Chemistry of Fragrances. Royal Society of Chemistry.
Rowe, D. J. (Ed.). (2005). Chemistry and Technology of Flavors and Fragrances. Blackwell Publishing.
Surburg, H., & Panten, J. (2016). Common Fragrance and Flavor Materials: Preparation, Properties and Uses (6th ed.). Wiley-VCH.
Symrise. (2022). Brahmanol—Product sheet & regulatory data. Symrise AG.
Turin, L. (2002). A method for the calculation of odor character from molecular structure. Journal of Theoretical Biology, 216(3), 367–385. https://doi.org/10.1006/jtbi.2002.3041
Yudov, M. (2017). Sandalwood in contemporary perfumery: From essential oil to Javanol. Fragrantica. Retrieved from https://www.fragrantica.com/news/Sandalwood-In-Contemporary-Perfumery-From-Essential-Oil-To-Javanol-10704.html