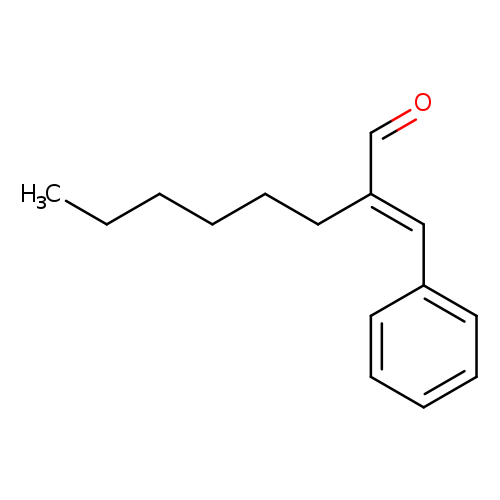
Jasmonal H ( α-Hexylcinnamaldehyde ) Technical Ingredient Overview
🔎 Chemical Name — (2E)-2-(Phenylmethylidene)octanal
🧪 Synonyms — α-Hexylcinnamaldehyde, Hexyl cinnamaldehyde, Hexyl cinnamal, Hexyl cinnamic aldehyde, α-Hexyl-β-phenylacrolein, α-n-Hexyl-β-phenylacrolein, 2-Benzylideneoctanal, 2-Hexyl-3-phenyl-2-propenal, Jasminolene
📂 CAS Number — 101-86-0
📘 FEMA Number — 2569
⚖️ Molecular Weight — 216.32 g/mol
📝 Odor Type — Floral, jasmine-like
📈 Odor Strength — Moderate to strong; tenacious with excellent longevity
👃🏼 Odor Profile — Very mild, sweet-oily, slightly floral odor with a trace of herbaceous undertones. The odor becomes considerably more floral and lively when incorporated with more volatile materials. Exhibits rich jasmine character with waxy-green, oily-herbaceous facets, sweet tropical nuances, and a soft powdery finish. Less sharp than amyl cinnamaldehyde with superior substantivity.
⚗️ Uses — Essential component in jasmine, gardenia, tuberose, magnolia, honeysuckle, and lily compositions. Widely used in fine fragrance, functional perfumery (soaps, detergents, shampoos), and as a FEMA-approved flavor ingredient in food applications. Functions as a fixative and floral modifier with excellent blending properties.
🧴 Appearance — Pale yellow to yellow liquid; may solidify to waxy solid at cooler temperatures. Commercial material typically contains stabilizers (e.g., BHT) to prevent oxidation.
What is Jasmonal H?
Jasmonal H, systematically known as α-hexylcinnamaldehyde (CAS 101-86-0), is a synthetic aromatic aldehyde belonging to the cinnamaldehyde family. It is characterized by a conjugated α,β-unsaturated aldehyde structure featuring a phenyl ring, an extended carbon chain, and an aldehyde functional group. This structural configuration imparts a distinctive floral-jasmine character that has made it an indispensable tool in perfumery for over seven decades.
As a semi-synthetic derivative, α-hexylcinnamaldehyde is produced via crossed aldol condensation of benzaldehyde with octanal, yielding predominantly the trans (E) isomer, which exhibits the most desirable olfactory properties (Wikipedia, 2024). While it occurs naturally in trace amounts in chamomile essential oil (Matricaria chamomilla), commercial material is exclusively synthetic due to economic and quality considerations (Sigma-Aldrich, n.d.).
Jasmonal H is classified within the broader aromatic aldehyde family, specifically as a substituted cinnamaldehyde. Its chemical formula is C₁₅H₂₀O, with a molecular weight of 216.32 g/mol (PubChem, n.d.). The material exhibits low water solubility but excellent solubility in organic solvents and lipophilic fragrance bases, making it compatible across diverse formulation matrices.
Historical Background
The development of synthetic jasmine components emerged as a critical objective in early 20th-century fragrance chemistry, driven by the prohibitive cost and limited availability of natural jasmine absolute. Among the most successful early synthetics were the α-alkylcinnamaldehydes, a homologous series of aldehydes that provided warm, floral-balsamic notes complementary to natural jasmine extracts.
α-Hexylcinnamaldehyde was first synthesized and commercialized in the mid-20th century as part of systematic research into cinnamaldehyde derivatives. Its synthesis follows classical organic chemistry principles: aldol condensation between benzaldehyde (C₆H₅CHO) and octanal (C₈H₁₆O) under basic catalysis, yielding the desired α,β-unsaturated aldehyde with high trans selectivity (Arctander, 1969). This reaction represented a scalable, cost-effective route to a material that could enhance and extend jasmine accords without the variability inherent in natural extracts.
By the 1950s and 1960s, α-hexylcinnamaldehyde had become a staple in both fine and functional perfumery. Renowned perfume materials authority Steffen Arctander provided extensive documentation of the material in his seminal 1969 work Perfume and Flavor Chemicals, describing its "very mild, sweet-oily, slightly floral odor with a trace of herbaceous undertones" and noting that "the odor becomes considerably more floral and lively when the material is incorporated with more volatile material in a composition" (Arctander, 1969, p. 110). Arctander emphasized its utility in jasmine, gardenia, tuberose, and magnolia bases, cementing its status as an essential building block.
The material gained further prominence through its inclusion in landmark fragrances of the mid-to-late 20th century. Its presence in compositions such as Christian Dior's Eau Sauvage (1966) and numerous jasmine-centered feminine fragrances established α-hexylcinnamaldehyde as a signature component of modern floral perfumery.
Olfactory Profile
Scent Family
Floral-Jasmine / Aldehydic-Floral
α-Hexylcinnamaldehyde is primarily classified within the floral-jasmine subfamily of aldehydic florals. It shares structural and olfactory kinship with related materials such as amyl cinnamaldehyde and benzyl cinnamate but is distinguished by its softer, more diffusive character and superior tenacity.
Main Descriptors
Primary Character: Sweet-oily floral with distinct jasmine tonality
Secondary Facets: Waxy-green, herbaceous-oily undertones
Tertiary Nuances: Soft powdery finish, tropical-fruity hints, mild balsamic warmth
The olfactory profile of α-hexylcinnamaldehyde is notable for its softness and lack of harshness compared to related cinnamaldehyde derivatives. While amyl cinnamaldehyde (the C₇ homolog) presents a fresher, more overtly floral character, hexyl cinnamaldehyde offers greater warmth, body, and persistence. The additional carbon in the alkyl chain provides enhanced lipophilicity, contributing to improved tenacity and smoother diffusion.
Arctander's description remains authoritative: the material exhibits a "very mild, sweet-oily, slightly floral odor" that gains vibrancy and floralcy when blended with more volatile top-note materials. This synergistic behavior makes it particularly valuable in jasmine reconstructions, where it functions as a binding agent that unifies disparate components into a cohesive bouquet (Arctander, 1969).
Intensity
Moderate-to-Strong with High Substantivity
α-Hexylcinnamaldehyde exhibits moderate initial diffusion but exceptional substantivity, remaining detectable on perfumer's blotters for over 400 hours (Fraterworks, 2025). This longevity makes it an effective fixative and base-note contributor in floral compositions.
Tenacity
Excellent (Base Note Substantivity)
The material demonstrates remarkable persistence on both skin and fabric, a property attributed to its relatively low volatility (boiling point approximately 308°C at 760 mmHg) and strong affinity for lipophilic substrates. This characteristic is particularly advantageous in functional perfumery applications such as fabric care and personal wash products, where long-lasting fragrance performance is critical.
Volatility
Low Volatility / Base-to-Middle Note
α-Hexylcinnamaldehyde functions primarily as a base note with heart-note characteristics when used in higher concentrations. Its low vapor pressure (approximately 0.0±0.6 mmHg at 25°C) ensures gradual evaporation, contributing to extended dry-down phases in fragrance compositions.
Fixative Role
Significant Fixative and Blending Agent
Beyond its intrinsic floral character, α-hexylcinnamaldehyde serves as an effective fixative that "assists in fixation of the fragrance" due to its tenacity (Arctander, 1969). It excels at tempering the sharp, volatile character of materials such as benzyl acetate, phenylethyl alcohol, and linalool, providing a smooth transition between top and base notes. Its blending properties are particularly valuable in complex floral bouquets, where it acts as a unifying agent that enhances overall cohesion and radiance.
Applications in Fine Fragrance
α-Hexylcinnamaldehyde occupies an essential position in the perfumer's palette as a foundational component of white floral accords. Its primary role is in jasmine reconstructions, where it provides the soft, oily-floral backdrop that characterizes natural jasmine absolute. Unlike more assertive materials such as indole or methyl anthranilate, which contribute the heady, animalic facets of jasmine, α-hexylcinnamaldehyde offers roundness, warmth, and substantivity without overwhelming delicacy.
Role in Fragrance Compositions
Jasmine Accords: α-Hexylcinnamaldehyde is an indispensable component of synthetic jasmine bases, where it functions alongside benzyl acetate, linalool, indole, and methyl anthranilate to recreate the multifaceted character of natural jasmine. It provides the creamy, waxy-green middle ground that balances sharper top notes and animalic undertones (Arctander, 1969).
Gardenia Compositions: In gardenia accords, the material contributes the rich, buttery-floral character that defines this flower's fragrance. It blends seamlessly with tuberose lactones, styrax, and benzyl salicylate to create lush, opulent white-floral effects.
Tuberose and Magnolia: The sweet, tropical-floral facets of α-hexylcinnamaldehyde complement the creamy, narcotic character of tuberose and the clean, citrus-tinged floralcy of magnolia. It serves as a bridge between green top notes and heavier base materials.
Muguet (Lily of the Valley) Bases: α-Hexylcinnamaldehyde features prominently in muguet reconstructions, where its fresh-floral character enhances the green-waxy profile of hydroxycitronellal, lilial (prior to restriction), and other muguet materials. Notable examples include its use in compositions such as Dior's Eau Sauvage (1966) and Chanel's Gabrielle (2017).
Typical Accords and Combinations
α-Hexylcinnamaldehyde pairs exceptionally well with:
Benzyl Acetate: Softens the sharp, fruity-floral character of benzyl acetate while enhancing jasmine authenticity
Linalool and Linalyl Acetate: Creates fresh-floral lift with herbaceous-green nuances
Indole and Methyl Anthranilate: Balances animalic, heady facets with creamy warmth
Hedione and Methyl Dihydrojasmonate: Provides body and tenacity to transparent jasmine frameworks
Ylang-Ylang and Tuberose Absolutes: Reinforces natural white-floral materials with synthetic substantivity
Hydroxycitronellal and Cyclamenaldehyde: Adds depth and warmth to lily-of-the-valley accords
Pairing Behavior with Other Ingredients
The material exhibits excellent compatibility across diverse fragrance families. Its soft, non-aggressive character allows for high loading levels (up to 10–15% in some compositions) without introducing harshness or dissonance. When combined with more volatile materials, α-hexylcinnamaldehyde enhances their floral character and extends their presence in the dry-down phase. Its ability to "temper the harsh character of benzyl acetate and other strong jasmine chemicals" makes it invaluable in achieving balance and refinement in complex white-floral compositions.
Performance in Formula
Behavior in Blends and Mixtures
α-Hexylcinnamaldehyde demonstrates exceptional blending behavior characterized by synergy with other floral materials. Its lipophilic nature ensures compatibility with both hydrophobic aroma chemicals and natural essential oils, facilitating uniform dispersion in alcoholic and oil-based fragrance systems. The material does not exhibit phase separation issues and maintains stability across a wide range of pH conditions, making it suitable for use in alkaline media such as soap perfumery (Arctander, 1969).
The material's oxidative sensitivity necessitates careful formulation practices. Unprotected α-hexylcinnamaldehyde is prone to autoxidation, leading to the formation of carboxylic acids and other degradation products that impart undesirable rancid, fatty, or cardboard-like off-notes. To mitigate this, commercial grades typically contain antioxidants such as butylated hydroxytoluene (BHT) at concentrations of 0.01–0.05% (Pell Wall, n.d.). Proper storage—preferably in cool, dark conditions with minimal air exposure—further extends shelf life and maintains olfactory integrity.
Impact on Overall Composition
Beyond its direct olfactory contribution, α-hexylcinnamaldehyde exerts several indirect effects on fragrance performance:
Fixative Effect: Its low volatility and high substantivity extend the longevity of more volatile floral materials, reducing top-note evaporation rates and enhancing overall tenacity.
Smoothing and Rounding: The material softens sharp edges in compositions, particularly when used alongside acetates, aldehydes, or high-impact green notes. This smoothing effect contributes to a perception of quality and refinement.
Volume and Body: α-Hexylcinnamaldehyde adds volume and fullness to floral accords without increasing intensity, creating a sense of richness and depth that enhances perceived complexity.
Alkaline Stability: Unlike many delicate floral materials, α-hexylcinnamaldehyde maintains its olfactory profile in alkaline formulations, making it highly suitable for soap perfumery and detergent applications (Arctander, 1969).
Typical usage levels range from 2–10% in fine fragrance concentrates, with exceptional cases reaching 15–30% in specialized jasmine or gardenia bases (Arctander, 1969). In functional perfumery, concentrations of 0.5–3% are common, providing substantive floral character without overwhelming consumer products.
Industrial & Technical Uses
Non-Fragrance Applications
Flavor Industry: α-Hexylcinnamaldehyde holds FEMA GRAS (Generally Recognized as Safe) status under FEMA number 2569, permitting its use as a flavoring agent in food and beverage applications. It imparts sweet, waxy-floral, rosy, and honey-like notes with fruity nuances, finding application in fruit flavors (particularly tropical and stone fruit profiles), floral-honey flavor systems, and confectionery products (Chem-Impex, n.d.).
Typical flavor use levels are significantly lower than in perfumery, generally ranging from 0.1–5 ppm depending on the application and desired intensity (Mosciano, 1997).
Industrial Processes and Functions
Synthesis and Manufacturing: The industrial synthesis of α-hexylcinnamaldehyde follows a classical crossed aldol condensation protocol:
Aldol Condensation: Benzaldehyde (C₆H₅CHO) reacts with octanal (C₈H₁₆O) in the presence of a base catalyst (typically sodium hydroxide or potassium hydroxide) at controlled temperatures (40–60°C).
Dehydration: The β-hydroxy aldehyde intermediate undergoes spontaneous or acid-catalyzed dehydration to yield the α,β-unsaturated aldehyde.
Purification: The crude product is purified via vacuum distillation, yielding material of >85–95% purity, with the trans (E) isomer predominating (>90%) (TCI America, n.d.).
Stabilization: Antioxidants (e.g., BHT) are added to prevent oxidative degradation during storage and handling.
Technical Specifications and Requirements
Purity: Commercial grades typically range from 85% (technical grade) to >97% (high-purity/analytical grades)
Refractive Index (n₂₀/D): 1.546–1.550
Density (20°C): Approximately 1.0 g/cm³
Boiling Point: 308.1°C at 760 mmHg
Flash Point: 140.5°C (closed cup)
Solubility: Insoluble in water; soluble in ethanol, diethyl phthalate, and other organic solvents
Storage: Store in tightly sealed containers, protected from light and air, preferably under refrigeration (Sigma-Aldrich, n.d.)
Regulatory & Safety Overview
IFRA Status
α-Hexylcinnamaldehyde is subject to regulation under the International Fragrance Association (IFRA) Standards due to its classification as a skin sensitizer. Under IFRA Amendment 48, the material is subject to restrictions based on dermal sensitization potential, with category-specific maximum use levels established to minimize allergic contact dermatitis risk (IFRA, 2020).
As of the 51st Amendment (effective June 2023), α-hexylcinnamaldehyde continues to be restricted in consumer products. While specific limits vary by product category, the material is generally permitted at concentrations that ensure the overall fragrance mixture does not exceed established Quantitative Risk Assessment (QRA) thresholds for sensitization (IFRA, 2023).
Official IFRA Documentation: IFRA Standard for α-Hexyl cinnamic aldehyde (Amendment 48+)
EU Cosmetics Regulation
Under EU Regulation (EC) No. 1223/2009 (Cosmetics Regulation), as amended by Regulation (EU) 2017/1410, α-hexylcinnamaldehyde is listed among the 26 regulated fragrance allergens in Annex III, Part 1. The material must be labeled on cosmetic product ingredient lists when present at concentrations exceeding:
0.001% in leave-on products
0.01% in rinse-off products
This labeling requirement enables consumers with known sensitivities to make informed purchasing decisions (European Commission, 2009).
INCI Name: Hexyl Cinnamal
FEMA Status
α-Hexylcinnamaldehyde is approved for use as a flavoring substance under FEMA Number 2569, with GRAS (Generally Recognized as Safe) status for food applications. It is recognized for use in baked goods, confectionery, beverages, and other food categories at levels consistent with good manufacturing practices (FEMA, n.d.).
Toxicology
α-Hexylcinnamaldehyde has been extensively evaluated for safety in both fragrance and flavor applications:
Skin Sensitization: The material is a recognized skin sensitizer, with positive reactions observed in patch testing of dermatological patients at an incidence rate of approximately 0.1% (Wikipedia, 2024). However, sensitization rates remain low in the general population when used within established regulatory limits.
Acute Toxicity: The material exhibits low acute toxicity by oral, dermal, and inhalation routes. No significant systemic toxicity has been observed at concentrations used in consumer products.
Environmental Toxicity: α-Hexylcinnamaldehyde is classified as hazardous to the aquatic environment, with chronic toxicity effects on aquatic organisms (Sigma-Aldrich, n.d.). Proper waste disposal and wastewater treatment protocols are necessary to minimize environmental impact.
Genotoxicity and Carcinogenicity: No evidence of genotoxic or carcinogenic potential has been identified in standard regulatory testing (RIFM, n.d.).
GHS Classification (EU CLP):
H317: May cause an allergic skin reaction
H410: Very toxic to aquatic life with long-lasting effects
Precautionary Statements:
Avoid release to the environment
Wear protective gloves when handling concentrated material
In case of skin contact, wash with soap and water; seek medical attention if irritation develops
References
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals) (Vols. I–II). Montclair, NJ: Author.
Chem-Impex. (n.d.). α-Hexylcinnamaldehyde (CAS 101-86-0) Product Information. Retrieved October 2025, from https://www.chemimpex.com/products/34375
European Commission. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Official Journal of the European Union, L342/59.
FEMA (Flavor and Extract Manufacturers Association). (n.d.). FEMA GRAS Assessment: Hexyl Cinnamaldehyde (FEMA 2569). Retrieved October 2025.
Fraterworks. (2025). Hexyl Cinnamic Aldehyde – Technical Data Sheet. Retrieved October 2025, from https://fraterworks.com/products/hexyl-cinnamic-aldehyde
International Fragrance Association (IFRA). (2020). IFRA Standard for α-Hexyl cinnamic aldehyde (Amendment 48). Geneva: IFRA International.
International Fragrance Association (IFRA). (2023). IFRA Standards – 51st Amendment. Geneva: IFRA International.
Mosciano, G. (1997). Organoleptic characteristics of flavor materials. Perfumer & Flavorist, 22(6), 41.
Pell Wall. (n.d.). Hexyl cinnamaldehyde – Technical Overview. Retrieved October 2025, from https://pellwall.com/products/hexyl-cinnamaldehyde
PubChem. (n.d.). α-Hexylcinnamaldehyde (CID 1550884) – Compound Summary. National Center for Biotechnology Information. Retrieved October 2025, from https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Hexylcinnamaldehyde
RIFM (Research Institute for Fragrance Materials). (n.d.). Safety Assessment: Hexyl Cinnamaldehyde. Woodcliff Lake, NJ: RIFM.
Scentspiracy. (2025). Jasmonal A / Amyl Cinnamal – Technical Overview. Retrieved October 2025, from https://www.scentspiracy.com/fragrance-ingredients/p/jasmonal-a
Sigma-Aldrich. (n.d.). α-Hexylcinnamaldehyde Analytical Standard (CAS 101-86-0). Retrieved October 2025.
TCI America. (n.d.). alpha-Hexylcinnamaldehyde (Product No. H0685). Retrieved October 2025, from https://www.tcichemicals.com/US/en/p/H0685
Wikipedia. (2024, September 26). Hexyl cinnamaldehyde. Retrieved October 2025, from https://en.wikipedia.org/wiki/Hexyl_cinnamaldehyde