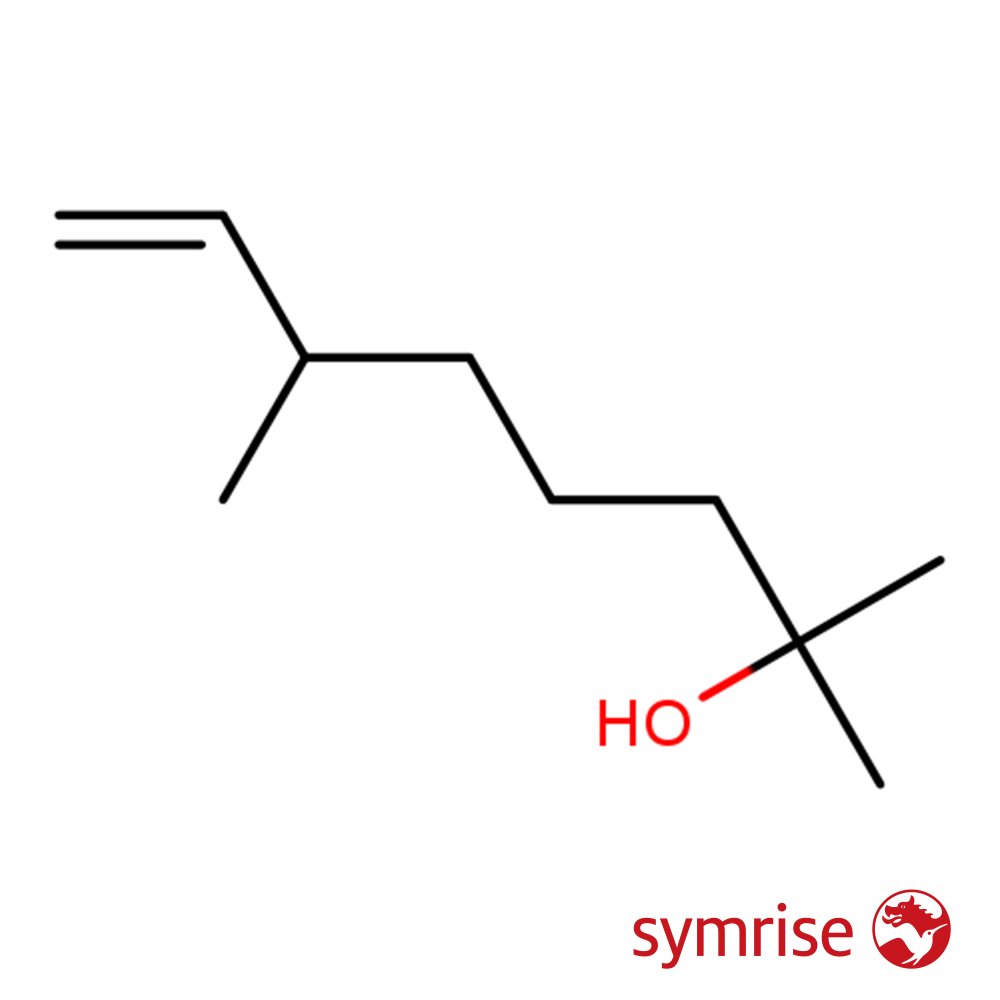
Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Gamma Nonalactone (Aldehyde C18)
Premium Synthetic Ingredient for Perfumery
Gamma-Nonalactone (Aldehyde C-18) is a high-impact synthetic lactone with an intense coconut-like aroma, widely used across perfumery and flavor applications. Despite its common trade name, it is not an aldehyde but a γ-lactone, offering creamy, musky, fruity warmth. It serves as a sweet, tropical enhancer in white florals and Oriental bases, with notable persistence and volume. Careful dosage is essential due to its tenacious character.
Premium Synthetic Ingredient for Perfumery
Gamma-Nonalactone (Aldehyde C-18) is a high-impact synthetic lactone with an intense coconut-like aroma, widely used across perfumery and flavor applications. Despite its common trade name, it is not an aldehyde but a γ-lactone, offering creamy, musky, fruity warmth. It serves as a sweet, tropical enhancer in white florals and Oriental bases, with notable persistence and volume. Careful dosage is essential due to its tenacious character.
Premium Synthetic Ingredient for Perfumery
Gamma-Nonalactone (Aldehyde C-18) is a high-impact synthetic lactone with an intense coconut-like aroma, widely used across perfumery and flavor applications. Despite its common trade name, it is not an aldehyde but a γ-lactone, offering creamy, musky, fruity warmth. It serves as a sweet, tropical enhancer in white florals and Oriental bases, with notable persistence and volume. Careful dosage is essential due to its tenacious character.
Synthetic Ingredient Overview
🔎 Chemical Name: γ-Nonalactone
🧪 Synonyms: Aldehyde C-18, Coconut Lactone
🧬 Chemical Formula: C₉H₁₆O₂
📂 CAS N°: 104-61-0
📘 FEMA: 2783
⚖️ MW: 156.22 g/mol
♨️ Vapor Pressure (25 °C): ~1.18 × 10⁻² mm Hg (est.)
📝 Odor Type: Fruity (Coconut)
📈 Odor Strength: Medium-strong, with extreme longevity (approx. 300 hrs on blotter)
👃🏼 Odor Profile: Intense coconut, creamy, fruity in high dilution, soft floral-musk undercurrent
👅 Flavor Profile: Coconut, sweet nutty, used in fruit and dessert-type flavors
⚗️ Uses: Floral volume builder, lactonic booster in white florals, coconut flavor agent, fixative
🧴 Appearance: Colorless to pale yellow liquid
What is Gamma-Nonalactone?
Gamma-Nonalactone, often mistakenly referred to as “Aldehyde C-18,” is a γ-lactone — a cyclic ester — derived from nonanoic acid. Despite the name confusion, it is chemically distinct from aldehydes. First reported in the 1920s and widely used since the 1950s, it became a staple in perfumery for its tropical, creamy, and long-lasting coconut character.
Structurally, it is a medium-sized ring lactone with significant volatility and diffusiveness for a base note compound. Its intense sweetness and tropical warmth allow it to function as both a fruity top enhancer and a long-wearing base material.
Olfactory Profile & Perfumery Applications
Gamma-Nonalactone adds unmistakable coconut warmth to both floral and Oriental compositions. Its use must be measured carefully: while trace amounts yield a creamy sweetness, even moderate overdosing can overpower compositions with lactonic heaviness.
Notable applications:
White Floral Bases: Enhances Gardenia, Jasmine, Tuberose, Honeysuckle, Plumeria
Oriental & Musky Accords: Used with Sandalwood, Styrax, Musk, and Undecanolide
Coconut Accords: Core component in beachy tropical profiles
Fixative Role: Contributes depth and longevity to fruity and creamy blends
Typical usage levels range between 0.01% and 0.3% in fine fragrance. In functional applications (candles, soaps), levels may be adjusted for thermal or surfactant resistance.
Industrial & Technical Uses
Fine Fragrance: Coconut-floral accords, exotic sweet notes, solar blends
Functional Fragrance: Long-lasting odor masking in detergents, personal care
Flavor Use (FEMA 2783): Used at 10–50 ppm in tropical, coconut, cherry, or nut flavors
Industrial Synthesis: Key building block in lactonic structures, low-odor solvents
Regulatory & Safety Overview
IFRA Status (2023): No specific restrictions; evaluated as standard lactone
EU Allergens (1223/2009): Not among 26 declarable allergens
REACH: Registered
ECHA Classification: Not classified as hazardous
FEMA GRAS Status: FEMA No. 2783 — Approved for flavor use
Toxicology: Low acute toxicity; not sensitizing under controlled levels
✅ Safe under normal usage conditions. Overuse may cause cloying odor profiles; not phototoxic or carcinogenic under typical perfumery exposure.
Natural Occurrence
Gamma-Nonalactone has been reported in a wide variety of natural matrices, including:
Fruits: Peach, Apricot, Papaya, Guava, Currant, Strawberry, Raisin
Fermented Products: Rum, Whiskey, Wine, Sherry, Beer
Animalic Foods: Chicken, Pork, Beef fat, Butter, Cheese
Others: Camembert, Tomato, Soybean, Green Tea, Rice, Malt, Sweetgrass
Despite these occurrences, commercial use is almost entirely synthetic, due to isolation cost.
Production Methods
From Nonanoic Acid: Lactonization via acid catalysis
Via Hexanol + Methyl Acrylate: Free-radical route using di-tert-butylperoxide
From Undecylenic Acid + Malonic Acid: Alternate multi-step esterification
These routes allow scalable, cost-effective industrial supply for both flavor and fragrance use.
Sources
Symrise Technical Literature
Perfume and Flavor Chemicals – S. Arctander (1969)
Fenaroli’s Handbook of Flavor Ingredients
Monographs on Fragrance Raw Materials – D. L. J. Opdyke (RIFM)
Leffingwell Chirality Database
PubChem – Compound CID 7792
Scentspiracy Internal Research – Fulvio Ciccolo (2024)