Synthetic Ingredient Overview
🏭 Manufacturer — Symrise
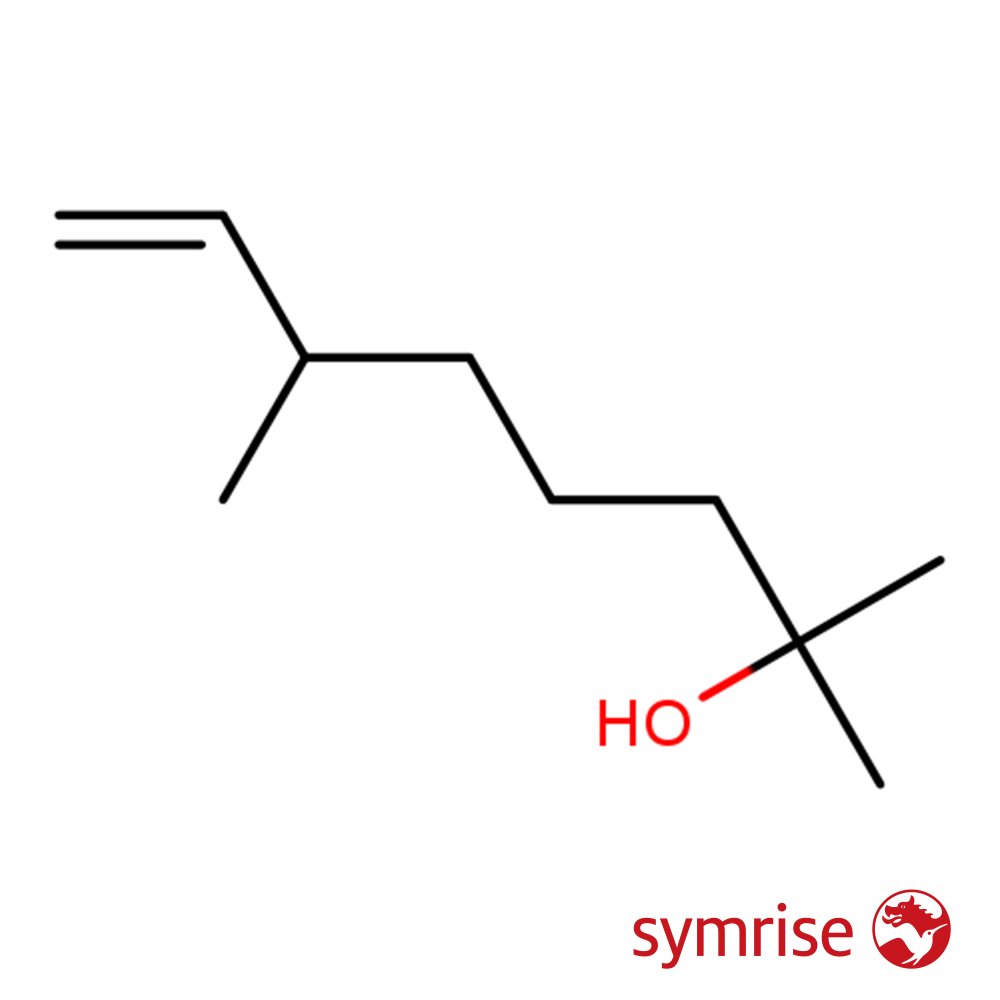
🔎 Chemical name — Dihydromyrcenol
📂 CAS N° — 18479-58-8
⚖️ MW — 156.26 g/mol
📝 Odor Type — Fresh (Citrus, Aromatic)
📈 Odor Strength — Weak (lingers several hours on blotter)
👃🏼 Odor Profile — Fresh, floral, lavender-like, slightly soapy and citrusy. Often compared to linalool. Clean, blue, household freshness, reminiscent of laundry detergents and glass cleaners.
👅 Flavor Profile — Green, aldehydic, herbal, terpenic, lime, tropical (not typically used in food)
⚗️ Uses — Extensively used in detergents, soaps, fougère fragrances, masculine colognes, aldehydic florals. Enhances top notes, adds brightness and coolness. Recommended at 1–5% in fragrance formulas.
🧴 Appearance — Clear, colorless to slightly viscous liquid
What is Dihydromyrcenol?
Dihydromyrcenol is a synthetic acyclic terpenoid alcohol with no known natural occurrence. It emerged in the early 1970s and became one of the most influential molecules in modern perfumery. First developed at IFF, it was incorporated into early masculine perfumes such as Paco Rabanne Pour Homme (1973) and Drakkar Noir (1982), where its ability to convey “hygienic freshness” redefined commercial scent aesthetics.
Chemically, Dihydromyrcenol is a mixture of approximately 50% 2,6-dimethyl-7-octen-2-ol and 50% 2,6-dimethyl-7-octen-2-yl formate. It is insoluble in water, slightly soluble in ethanol, and fully miscible in fragrance oils. It is typically produced via chlorination and hydrolysis of Dihydromyrcene or esterification followed by hydrolysis.
Olfactory Profile and Perfumery Applications
With its citrus-lavender hybrid profile, Dihydromyrcenol sits between top and heart notes, imparting an unmistakable clean-air freshness. Though considered weak in odor strength, its diffusion and longevity make it indispensable in large-scale fragrance systems. It performs well with:
Lavender, citrus, aldehydes, white musks
Floral ozonic bases (e.g., Davidoff Cool Water, 1988 – ~20% concentration)
Fougère structures (e.g., Azarro Pour Homme, Drakkar Noir)
Its scent character is so distinctive that overuse may lead to a formula being associated with household products or laundry detergent accords. Still, it remains highly effective at modulating harsh notes and extending the lifespan of volatile top materials like citrus esters or linalyl acetate.
Industrial and Technical Uses
Dihydromyrcenol is used at industrial scale in:
Detergents and fabric softeners
Toilet soaps and shampoos
Deodorants and body sprays
Air care products and room sprays
Its use exceeds 1,000 metric tons per year globally (IFRA, 2004), and it is valued for its cost-effectiveness, safety, and wide consumer acceptance.
Regulatory and Safety Overview
IFRA Standards: Allowed in most product categories. Follow IFRA guidance for skin contact applications.
ECHA Classification:
H315 – Causes skin irritation
H319 – Causes serious eye irritation
H411 – Toxic to aquatic life with long-lasting effects
GHS Phrases: P264, P273, P280, P302+P352, P305+P351+P338
REACH: Fully registered in the EU
FDA/FEMA: Not listed for food use
Phototoxicity: Not reported
Dermal Sensitization: Low sensitization potential; tested at 6% with no irritation reported
✅ Overall, Dihydromyrcenol is considered safe under regulated use, with no endocrine disruptor alerts.
Additional Notes
Olfactory Category Impact: Dihydromyrcenol is emblematic of the "New Freshness" wave and is now considered a benchmark of modern masculine olfactive architecture.
Formulation Note: Avoid overuse in premium fine fragrances to prevent a “detergent-like” perception.