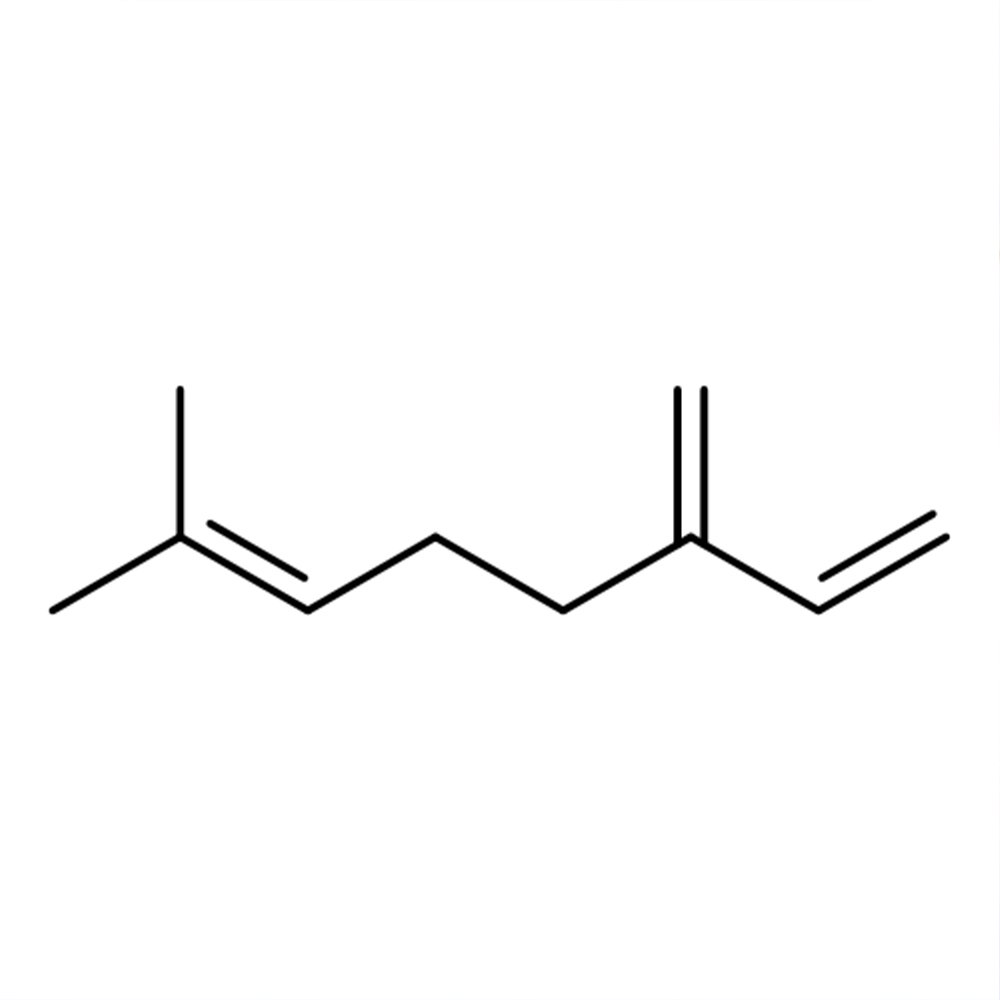
Myrcene (β-Myrcene) Technical Ingredient Overview
🔎 Chemical Name — 7-Methyl-3-methylene-1,6-octadiene
🧪 Synonyms — β-Myrcene, beta-myrcene, 2-methyl-6-methylene-2,7-octadiene, 3-methylene-7-methyl-1,6-octadiene, β-geraniolene
📂 CAS Number — 123-35-3
📘 FEMA Number — 2762 (GRAS status for flavor use)
⚖️ Molecular Weight — 136.23 g/mol
🧬 Molecular Formula — C₁₀H₁₆
📝 Odor Type — Green, balsamic, fruity-resinous
📈 Odor Strength — Medium to strong (volatile top note character)
👃🏼 Odor Profile — Sweet-balsamic and fruity-resinous with green mango nuances, light citrus notes, and slightly ethereal, mushroomy undertones. Fresh and volatile in purified form with poor tenacity
⚗️ Uses — Fragrance intermediate, extender for bay leaf oil and citrus accords, masking agent in industrial products, precursor for terpene alcohols (geraniol, nerol, linalool, citronellol), aldehydes (citral, citronellal), and menthol
🧴 Appearance — Pale yellow to colorless mobile liquid
What is Myrcene?
Myrcene (β-myrcene) is an acyclic monoterpene hydrocarbon naturally occurring in numerous essential oils but rarely extracted directly from botanical sources due to chemical instability. As a conjugated diene with three double bonds (including one conjugated system), myrcene is highly reactive and susceptible to oxidation and polymerization upon air exposure (Kolicheski et al., 2007).
The commercial material is semi-synthetic, produced predominantly through high-temperature pyrolysis of β-pinene derived from turpentine distillation. While myrcene exists naturally in over 200 plant species—including cannabis (29-66%), hops, bay leaf, lemongrass, myrtle, and cardamom—industrial-scale extraction from these sources remains economically unfavorable compared to synthesis (Surendran et al., 2021).
Chemically classified under IUPAC nomenclature as 7-methyl-3-methylene-1,6-octadiene, myrcene serves primarily as a key intermediate in terpene-based aroma chemistry rather than as a finished fragrance material, though it contributes characteristic peppery and balsamic notes to beer and certain flavor applications.
Historical Background
The systematic study of myrcene began in the early 20th century as part of broader investigations into terpene chemistry. While naturally occurring myrcene was identified in various essential oils, including bay leaf (Pimenta racemosa) and verbena, early researchers recognized the practical limitations of isolation from botanical sources.
The commercial viability of myrcene transformed significantly with the development of thermal pyrolysis methods for β-pinene conversion. By the mid-20th century, the pyrolysis process operating at approximately 400°C became the industry-standard synthesis route, enabling large-scale production from renewable turpentine feedstocks—waste materials from paper mill operations (Kolicheski et al., 2007).
Research into optimization of pyrolysis conditions has continued, with studies demonstrating theoretical yields of up to 93.5% myrcene from β-pinene, though practical industrial yields typically range from 75-85% due to side reactions and decomposition pathways (Kolicheski et al., 2007). The thermal isomerization mechanism proceeds through biradical intermediates, with competing reactions producing limonene and 1[7],8-p-menthadiene as byproducts.
Myrcene’s role evolved from a curiosity in essential oil analysis to a cornerstone intermediate in industrial terpene chemistry, particularly valued for downstream synthesis of high-value aroma chemicals and vitamin precursors.
Olfactory Profile
Scent Family
Terpenic/Green – Classified as an acyclic monoterpene hydrocarbon within the terpenic family
Main Descriptors
Primary character: Sweet-balsamic, fruity-resinous with pronounced green facets
Secondary notes: Mango-like fruitiness, light citrus nuances, slightly ethereal quality
Tertiary aspects: Subtle mushroomy undertones, peppery and vegetable-like characteristics
Quality variations: Fresh and volatile when purified; can develop harsh, aggressive notes in oxidized or polymerized states
Intensity
Medium to strong odor strength, though the pure material displays volatility that limits its substantivity in finished compositions
Tenacity
Poor to very poor – Highly volatile top note character with minimal fixative properties. Evaporates rapidly and contributes little to the dry-down phase of fragrances
Volatility
Extremely high – Functions exclusively as a top note material. Rapid evaporation rate characteristic of light monoterpenes. Flash point below 50°C (122°F)
Fixative Role
Myrcene possesses no fixative properties and is typically used as an extender or modifier rather than for longevity enhancement. Its reactive conjugated diene structure makes it unsuitable for applications requiring stability
Applications in Fine Fragrance
In fine fragrance, myrcene serves primarily as a functional ingredient rather than a featured note. Its applications include:
Extender for natural oils: Particularly effective for bay leaf oil, citrus oils, and spice colognes where it adds lightness and freshness
Green modifiers: Contributes natural-feeling herbaceous and vegetable-like facets to compositions without introducing harsh chemical notes
Citrus accords: Enhances brightness and natural character in lemon, lime, and mixed citrus formulations
Masking agent: Modifies overly sharp or fatty base notes in functional fragrances
Typical usage levels: 0.5-5% in fine fragrance concentrates, though often lower due to volatility concerns
Common pairings: Citrus oils (bergamot, lemon, lime), pine needle, bay leaf, cardamom, ginger, lavender, geranium, and other terpenic materials
Performance in Formula
Stability considerations: Myrcene’s conjugated diene structure makes it prone to oxidation and auto-polymerization. Commercial grades require stabilization with antioxidants such as butylated hydroxytoluene (BHT), alkylphenols, or tocopherols to prevent degradation.
Reactivity: Can undergo Diels-Alder reactions with various dienophiles, forming cyclohexene derivatives—a characteristic exploited intentionally in the synthesis of materials like Lyral® (4-(4-hydroxy-4-methylpentyl)-3-cyclohexene-1-carboxaldehyde).
Formulation behavior: Blends readily with alcoholic and hydrocarbon solvents. May exhibit poor stability in alkaline bases (soaps) and certain candle wax systems where its odor becomes imperceptible or distorted.
Industrial & Technical Uses
Beyond perfumery, myrcene functions as a critical chemical intermediate:
Terpene alcohol synthesis: Precursor to geraniol, nerol, linalool, and citronellol through acid-catalyzed hydration and rearrangement
Aldehyde production: Oxidative transformations yield citral, citronellal, and hydroxycitronellal
Menthol synthesis: Serves as starting material in industrial L-menthol production routes
Vitamin synthesis: Key intermediate in manufacturing vitamins A and E
Polymer chemistry: Used in specialty polymer applications including bio-based resins and sustainable materials research
Regulatory & Safety Overview
IFRA Status
Permitted without restriction as of IFRA Amendment 51 (notified June 30, 2023). No specific IFRA Standard exists limiting myrcene use in fragrance applications. However, formulators should consider:
Polymerization risks in finished products
Potential oxidation leading to sensitizing degradation products
Volatility affecting odor stability
IFRA Standards Library: https://ifrafragrance.org/standards-library
EU Cosmetics Regulation
Not listed among the 26 allergenic fragrance substances requiring declaration under EU Cosmetics Regulation (EC) No 1223/2009, Annex III. Compliant for use in cosmetic products when incorporated via fragrance compounds meeting overall regulatory requirements.
FEMA Status
FEMA No. 2762 – Granted Generally Recognized As Safe (GRAS) status for flavor applications. However, the U.S. FDA withdrew authorization for myrcene as a synthetic flavoring substance in October 2018, though this action was stated to be without regard to safety concerns under intended use conditions.
California Proposition 65
Added to California’s Proposition 65 list in 2015 as a chemical known to the State of California to cause cancer or reproductive harm, requiring warning labels for products sold in California containing myrcene above threshold levels.
Toxicology
Acute toxicity: Low acute toxicity by oral and dermal routes under normal use conditions
Skin sensitization: Myrcene itself shows minimal sensitization potential; however, oxidation products formed during storage or air exposure may exhibit sensitizing properties. Stabilization with antioxidants is recommended for commercial formulations (Surendran et al., 2021).
Systemic effects: Safe at standard industry usage levels. Demonstrates antioxidant properties in some biological models.
Environmental classification: Classified as hazardous to aquatic organisms with long-lasting effects (Aquatic Acute 1, Aquatic Chronic 1 under CLP/GHS)
Other hazards: Flammable liquid (Category 3); aspiration hazard (Category 1); may cause eye and skin irritation
References
Arctander, S. (1969). Perfume and flavor materials of natural origin. Elizabeth, NJ: Published by the author.
Bauer, K., Garbe, D., & Surburg, H. (2001). Common fragrance and flavor materials: Preparation, properties and uses(4th ed.). Weinheim: Wiley-VCH.
Kolicheski, M. B., Cocco, L. C., Mitchell, D. A., & Kaminski, M. (2007). Synthesis of myrcene by pyrolysis of β-pinene: Analysis of decomposition reactions. Journal of Analytical and Applied Pyrolysis, 80(1), 92-100. https://doi.org/10.1016/j.jaap.2007.01.007
National Center for Biotechnology Information. (2025). PubChem Compound Summary for CID 31253, Myrcene. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Myrcene
Pybus, D., & Sell, C. (Eds.). (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Cambridge: Royal Society of Chemistry.
Surendran, S., Qassadi, F., Surendran, G., Lilley, D., & Heinrich, M. (2021). Myrcene—What are the potential health benefits of this flavouring and aroma agent? Frontiers in Nutrition, 8, 699666. https://doi.org/10.3389/fnut.2021.699666