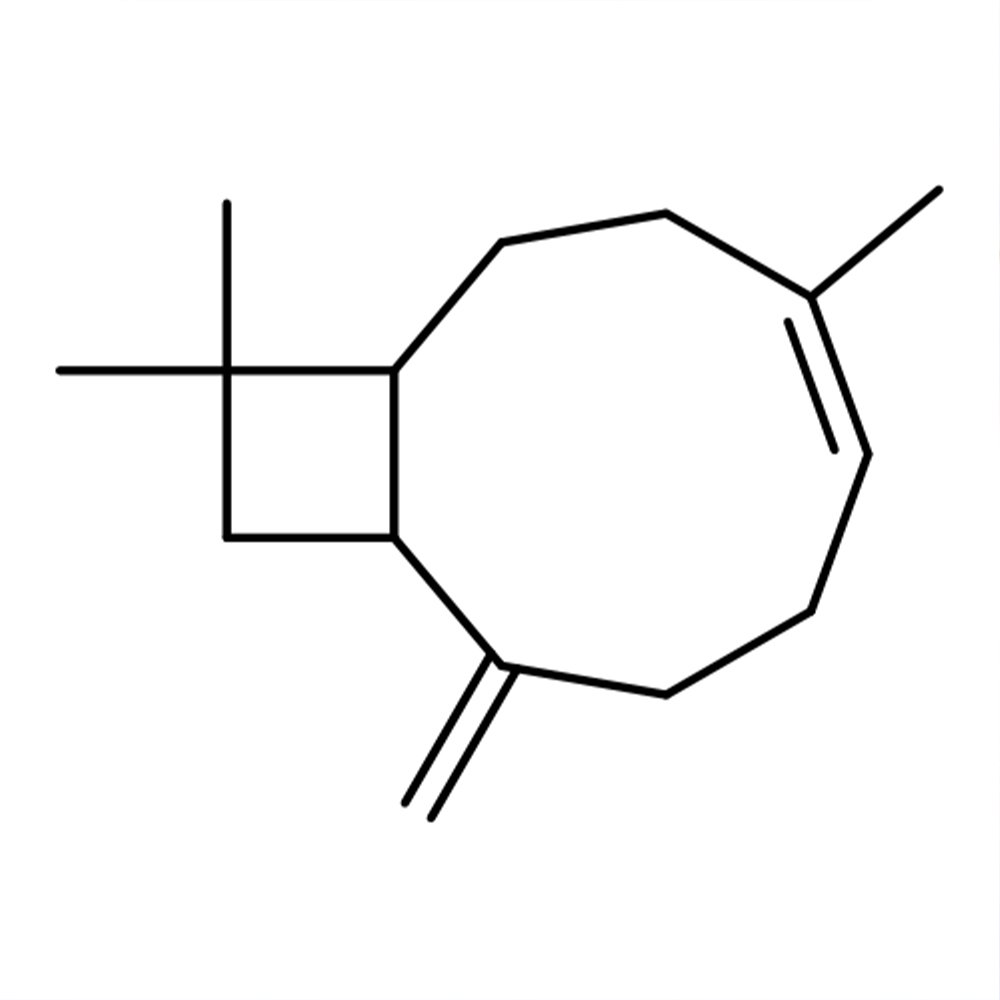
β-Caryophyllene (CAS 87-44-5) Technical Ingredient Overview
🔎 Chemical Name — (−)-β-Caryophyllene; (1R,4E,9S)-(−)-4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene
🧪 Synonyms — trans-Caryophyllene, (E)-Caryophyllene, β-(E)-Caryophyllene, trans-β-Caryophyllene, BCP, Caryophyllene B
📂 CAS Number — 87-44-5 (primary); 13877-93-5 (mixture including isomers)
📘 FEMA Number — 2252
⚖️ Molecular Weight — 204.35 g/mol
📝 Odor Type — Woody-spicy, terpenic
📈 Odor Strength — Medium to strong
👃🏼 Odor Profile — Woody-spicy with dry, clove-like character; tenacious opening with penetrating spicy notes transitioning to musty, driftwood nuances on drydown; slightly camphoraceous with pepper-like undertones
⚗️ Uses — Fragrance ingredient (top note), flavor additive, fixative for volatile spice chemicals, intermediate for synthesis
🧴 Appearance — Colorless to pale yellow clear liquid
What is β-Caryophyllene?
β-Caryophyllene is a natural bicyclic sesquiterpene that occurs widely in nature and is a constituent of many essential oils, particularly clove oil, black pepper, cannabis, rosemary, and hops. It is notable for having a cyclobutane ring as well as a trans-double bond in a 9-membered ring, both rarities in nature.
The compound belongs to the sesquiterpene class of natural products, meaning it contains 15 carbon atoms (C₁₅H₂₄) arranged in a complex bicyclic structure. Caryophyllene is one of the chemical compounds that contributes to the aroma of black pepper. The molecule exists naturally as the (−)-enantiomer and is typically found alongside its cis-isomer (isocaryophyllene) and the ring-opened isomer α-humulene.
Historical Background
The first total synthesis of caryophyllene in 1964 by E. J. Corey was considered one of the classic demonstrations of the possibilities of synthetic organic chemistry at the time. This landmark achievement by Elias James Corey, who later received the Nobel Prize in Chemistry in 1990, utilized innovative photochemical [2+2] cycloaddition reactions to construct the unusual cyclobutane ring system (Corey, 1963).
The synthesis was particularly significant because it demonstrated advanced retrosynthetic analysis and strategies for constructing medium-sized rings and strained ring systems—challenges that had limited synthetic organic chemistry prior to this work. The successful synthesis validated theoretical approaches to complex molecule construction and established methodologies that influenced organic synthesis for decades.
While β-caryophyllene has been known as a natural constituent of spices for centuries, its structure elucidation and synthetic accessibility marked a turning point in understanding sesquiterpene chemistry. Caryophyllene can be produced synthetically, but it is invariably obtained from natural sources because it is widespread.
Olfactory Profile
Scent Family
Classification: Woody-Spicy, Terpenic
Chemical Family: Bicyclic sesquiterpene hydrocarbons
Main Descriptors
β-Caryophyllene exhibits a distinctive woody-spicy aroma characterized by:
Primary: Dry, woody, clove-like character
Secondary: Spicy, peppery, slightly camphoraceous, Musty, driftwood, earthy undertones
Character: Tenacious, penetrating initially; transitions to softer woody-balsamic notes
Intensity
Medium to strong odor intensity with assertive opening notes that moderate over time. The initial perception is penetrating and characteristic, demanding attention in compositions.
Tenacity
Moderate tenacity with longevity of approximately 3-4 hours on a blotter. Despite its assertive opening, the compound exhibits faster evaporation than its woody character might suggest, positioning it as a transitional note between top and middle.
Volatility
Functions primarily as a top note with some middle note characteristics. The relatively high volatility places it in the more ephemeral category despite its woody-spicy profile, which is unusual for sesquiterpenes that typically exhibit middle to base note behavior.
Fixative Role
β-Caryophyllene serves as an effective fixative for more volatile spice chemicals. It acts as a fixative for more volatile spice chemicals, such as cinnamic aldehyde, stabilizing flavor profiles. This dual functionality—contributing its own character while anchoring lighter materials—makes it valuable in both fragrance and flavor applications.
Applications in Fine Fragrance
β-Caryophyllene plays a specialized role in perfumery, particularly in compositions requiring authentic spice and woody character. Primarily utilized as a top note, caryophyllene contributes to the spiciness and warmth in fragrance compositions.
Primary Applications:
Spice Accords: Essential for clove, pepper, and warm spice reconstructions
Woody Accords: Contributes dry, dusty wood character to cedar and sandalwood compositions
Oriental Fragrances: Provides authentic spice warmth in amber and incense structures
Fougère Compositions: Adds aromatic-spicy complexity to traditional lavender-coumarin structures
Synergistic Pairings:
Caryophyllene shares olfactory characteristics with clove leaf oil and cinnamic aldehyde, both contributing spicy and warm notes to fragrance compositions. It also complements humulene, another sesquiterpene found in hops and cannabis, known for its woody and earthy aroma.
Additional complementary materials include:
Oakmoss products and oakmoss absolutes
Geranium oils and geranyl derivatives
Vetiver and patchouli
Eugenol and isoeugenol
Natural spice oils (cinnamon, nutmeg, cardamom)
Performance in Formula
β-Caryophyllene exhibits excellent blending characteristics and stability in most perfume bases. Its chemical structure provides good resistance to oxidation compared to many terpenes, though beta-caryophyllene started to oxidize immediately when air exposed and after 5 weeks almost 50% of the original compound was consumed, with caryophyllene oxide found to be the major oxidation product.
Formulation Considerations:
Solubility: Miscible with most perfume materials and alcohol; insoluble in water
Stability: Generally stable in alcoholic solutions; sensitive to strong acids and bases
Compatibility: Blends well with both natural and synthetic materials
Dosage: Effective at 0.5-5% in fine fragrance; higher concentrations (up to 200 ppm) in flavor applications
pH Sensitivity: Not recommended for use in highly alkaline formulations such as soap due to terpene polymerization tendencies
Impact on Composition:
The compound introduces a characteristic dryness and natural spice authenticity that cannot be easily replicated by purely synthetic materials. Its moderate substantivity helps bridge volatile top notes with heavier base notes.
Industrial & Technical Uses
Beyond perfumery, β-caryophyllene demonstrates significant versatility across multiple industries.
Flavor Industry:
Caryophyllene finds applications in flavor compositions, mainly in spice blends and particularly in chewing gum, where concentrations may reach up to 200 ppm. It is recognized with FEMA GRAS Number 2252 as safe for use in flavor applications.
Food Additive Status:
Caryophyllene has been given generally recognized as safe (GRAS) designation by the FDA and is approved by the FDA for use as a food additive, typically for flavoring.
Pharmaceutical Research:
β-Caryophyllene is under basic research for its potential action as an agonist of the cannabinoid receptor type 2 (CB2 receptor). Recent studies have explored its anti-inflammatory, neuroprotective, and analgesic properties, though these applications remain in research stages.
Chemical Intermediate:
The compound serves as a starting material for synthesis of caryophyllene oxide and related derivatives used in specialty chemical applications.
Regulatory & Safety Overview
IFRA Status
β-Caryophyllene has no restrictions under IFRA Standards through Amendment 51. The RIFM safety assessment concluded that the existing information supports the use of this material, with β-caryophyllene found not to be Persistent, Bioaccumulative, and Toxic (PBT) as per IFRA Environmental Standards.
IFRA Standards Library: https://ifrafragrance.org/standards-library
EU Cosmetics Regulation
Beta-caryophyllene is classified as an allergen under European Regulation 2023/1545 dated July 26, 2023. Its presence must be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
Transition Timeline:
July 31, 2026: Compliance required for new products entering EU market
July 31, 2028: Compliance required for all products on EU market
FEMA Status
FEMA Number 2252; JECFA Number 1324. Approved as a flavoring agent with GRAS status.
Toxicology
RIFM evaluation showed that β-caryophyllene is not genotoxic, with calculated Margin of Exposure (MOE) >100 for repeated dose toxicity and fertility endpoints. Data show no safety concerns for skin sensitization under current declared levels of use. The compound is not expected to be phototoxic or photoallergenic.
Rats given up to 700 mg/kg daily for 90 days did not produce any significant toxic effects. Caryophyllene has an LD₅₀ of 5,000 mg/kg in mice.
Sensitization Note: Beta-caryophyllene itself is non-sensitizing, but caryophyllene oxide has been shown to be an allergen of moderate strength, and beta-caryophyllene air exposed for 10 weeks showed weak sensitizing capacity in the local lymph node assay. This underscores the importance of proper storage and handling to minimize oxidation.
Related Scentspiracy Materials
β-Caryophyllene shares chemical and olfactory relationships with several materials in the Scentspiracy database:
Cinnamic Aldehyde — Complementary spicy note requiring fixation
Eugenol — Clove constituent sharing spicy profile
Clove Leaf Oil — Natural source containing β-caryophyllene
Black Pepper Oil — Essential oil rich in β-caryophyllene
These connections enhance the depth and complexity of spice and woody accords in perfumery formulations.
Sources
Adams, T. B., Gavin, C. L., McGowen, M. M., Waddell, W. J., Cohen, S. M., Feron, V. J., Marnett, L. J., Munro, I. C., Portoghese, P. S., Rietjens, I. M. C. M., & Smith, R. L. (2011). The FEMA GRAS assessment of aliphatic and aromatic terpene hydrocarbons used as flavour ingredients. Food and Chemical Toxicology, 49(10), 2471-2494.
Api, A. M., Belsito, D., Biserta, S., Botelho, D., Bruze, M., Burton, G. A., Jr., Buschmann, J., Cancellieri, M. A., Dagli, M. L., Date, M., Dekant, W., Deodhar, C., Fryer, A. D., Jones, L., Joshi, K., Kumar, M., Lapczynski, A., Lavelle, M., Liebler, D. C., ... Tokura, Y. (2021). RIFM fragrance ingredient safety assessment, β-caryophyllene, CAS Registry Number 87-44-5. Food and Chemical Toxicology, 159(Suppl 1), Article 112425. https://doi.org/10.1016/j.fct.2021.112425
Corey, E. J. (1963). Total synthesis of d,l-caryophyllene and d,l-isocaryophyllene. Journal of the American Chemical Society, 85(3), 362-363. https://doi.org/10.1021/ja00886a037
European Commission. (2023). Commission Regulation (EU) 2023/1545 of 26 July 2023 amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as regards labelling of fragrance allergens in cosmetic products. Official Journal of the European Union, L 188/1.
Flavor and Extract Manufacturers Association. (n.d.). Beta-caryophyllene. FEMA Flavor Library. https://www.femaflavor.org/flavor-library/beta-caryophyllene
Karlberg, A. T., Magnusson, K., & Nilsson, U. (2005). The fragrance chemical beta-caryophyllene-air oxidation and skin sensitization. Food and Chemical Toxicology, 43(4), 511-517. https://doi.org/10.1016/j.fct.2004.12.001
National Center for Biotechnology Information. (2025). PubChem Compound Summary for CID 5281515, (−)-Caryophyllene. https://pubchem.ncbi.nlm.nih.gov/compound/Caryophyllene
U.S. Food and Drug Administration. (n.d.). Beta-caryophyllene. Substances Added to Food. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/
World Health Organization. (2006). Safety evaluation of certain food additives prepared by the sixty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series No. 54.