NEROLIONE Technical Ingredient Overview
🏭 Manufacturer — Symrise (primary commercial source)
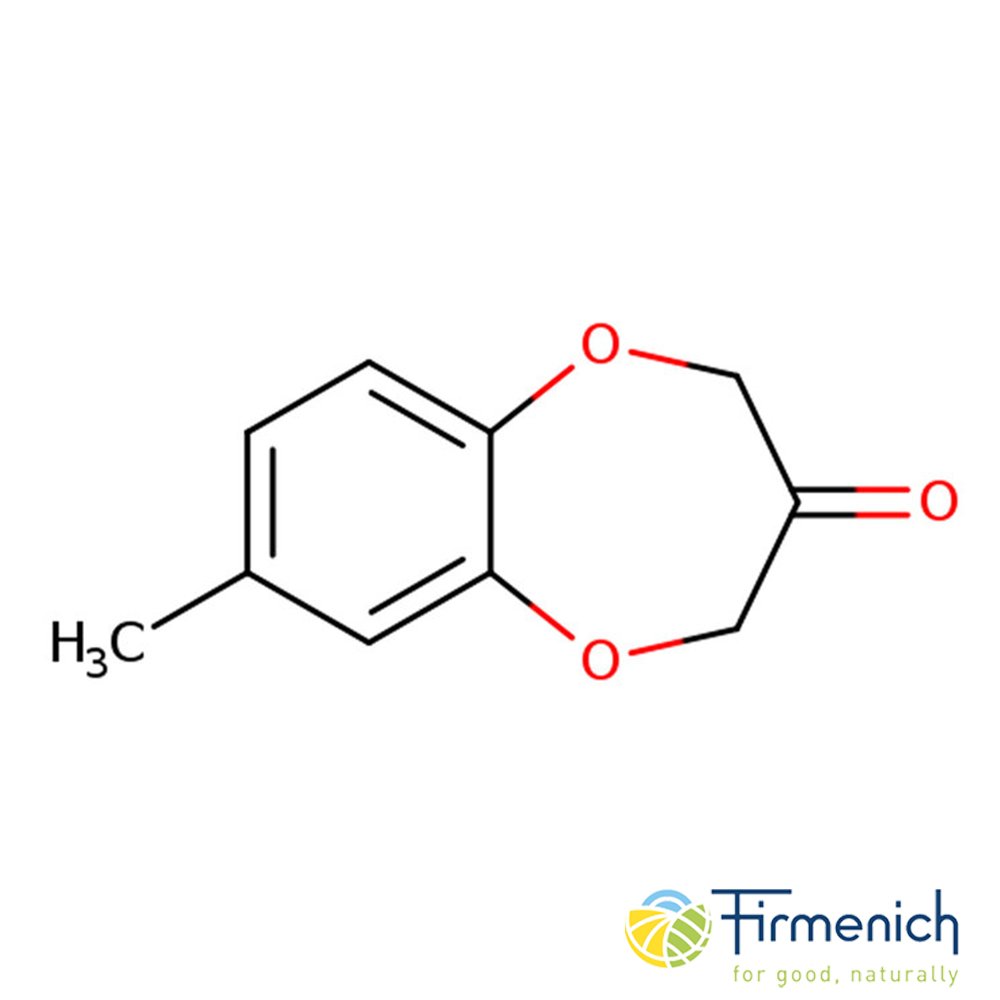
🔎 Chemical Name — 1-(3-methyl-1-benzofuran-2-yl)ethanone
🧪 Synonyms — Ethanone, 1-(3-methyl-2-benzofuranyl)-; 3-Methyl-2-acetylfuran; Methyl 3-methylbenzofuran-2-yl ketone
📂 CAS Number — 23911-56-0
📘 FEMA Number — Not assigned
⚖️ Molecular Weight — 174.19 g/mol
📝 Odor Type — Floral (Middle note)
📈 Odor Strength — Medium to strong (lasting 400+ hours on smelling strip)
👃🏼 Odor Profile — Floral, orange blossom character with light, delicate nuances; sweet neroli-like facets with green undertones
⚗️ Uses — Fine fragrance compositions, orange blossom reconstructions, floral accords, neroli bases
🧴 Appearance — Colorless to pale yellow liquid; can crystallize at room temperature
What is Nerolione?
Nerolione is a synthetic aromatic compound belonging to the benzofuran family of fragrance materials. Chemically classified as an acetylated benzofuran derivative, it represents an important tool in perfumery for recreating orange blossom and neroli notes. The molecule features a benzofuran ring system with a methyl substituent and an acetyl functional group, providing both aromatic character and carbonyl reactivity that contributes to its distinctive olfactory properties.
As a middle note ingredient, Nerolione exhibits excellent substantivity and tenacity, making it valuable in compositions requiring lasting floral impressions. Its chemical structure allows it to blend effectively with both natural essential oils and synthetic aroma chemicals, serving as a bridge between fresh top notes and deeper base notes in fragrance architecture.
Historical Background
Nerolione was developed as part of the ongoing search for synthetic alternatives to natural neroli oil and orange blossom absolute, which are expensive and subject to supply variability. The compound was synthesized and commercialized by Symrise (formerly Haarmann & Reimer) as a specialty material for high-quality perfumery applications.
The development of benzofuran-based fragrance materials emerged from systematic structure-activity relationship studies in the mid-to-late 20th century, when perfumery chemists explored heterocyclic compounds as sources of floral character. The name "Nerolione" directly references its olfactory similarity to neroli, the steam-distilled essential oil from bitter orange blossoms (Citrus aurantium subsp. amara).
Olfactory Profile
Scent Family: Floral (White Floral subcategory)
Main Descriptors: Floral, orange blossom, neroli-like, sweet, light, delicate, slightly green, fresh
Intensity: Medium to strong with excellent radiance; detectable at low concentrations but not overpowering
Tenacity: Excellent longevity (400+ hours on smelling strip); performs as a true middle note with gradual evolution
Volatility: Middle note character with moderate evaporation rate; bridges fresh top notes and substantive base notes
Diffusion: Good diffusivity with elegant projection; creates a soft halo effect rather than aggressive sillage
The odor profile of Nerolione closely mimics aspects of natural neroli oil, particularly the delicate, light floral facets found in high-quality orange blossom. Unlike the more pungent indolic aspects of some white florals, Nerolione maintains a clean, luminous character that reads as fresh and modern. The benzofuran structure imparts a slightly woody-aromatic undertone that adds depth and complexity to its floral presentation.
Applications in Fine Fragrance
Nerolione finds primary use in creating neroli and orange blossom accords where a lighter, more refined character is desired compared to the sometimes heavy sweetness of orange blossom absolute. It blends exceptionally well with other floral materials including jasmine, ylang-ylang, and tuberose, as well as with citrus notes (petitgrain, bergamot, bitter orange) and fresh green materials.
The material is particularly valuable in eau de cologne formulations, Mediterranean-inspired fragrances, and modern interpretations of classic floral themes. Its clean character makes it suitable for functional fragrance applications in personal care products where a sophisticated floral note is desired without excessive sweetness or indolic aspects.
Performance in Formula
In perfume formulations, Nerolione demonstrates excellent stability and compatibility with both alcoholic and oil-based systems. Its chemical structure provides good resistance to oxidation compared to some terpene alcohols, though standard antioxidant practices remain advisable. The material shows good substantivity on skin and textiles, contributing to the longevity of fragrance compositions.
Typical usage levels range from 0.1% to 4.0% in fine fragrance concentrations, with lower percentages (0.05-1.0%) in functional applications. The material exhibits some solubility limitations in highly aqueous systems but performs well in emulsions and typical fragrance carrier systems.
Industrial & Technical Uses
Beyond fine fragrance, Nerolione appears in premium personal care products including body lotions, shower gels, and hair care formulations where its orange blossom character adds luxury positioning. Home fragrance applications including candles and diffusers utilize Nerolione for its substantive floral character, though thermal stability should be evaluated for specific burning conditions.
The material's chemical stability profile makes it suitable for a range of consumer product matrices, though formulation testing is always recommended given the complexity of modern product systems.
Regulatory & Safety Overview
IFRA Status: Not specifically restricted under IFRA Amendment 51; usage should follow general safety guidelines for fragrance materials
EU Cosmetics Regulation: Compliant for use in cosmetic products when following good manufacturing practices and concentration limits
FEMA Status: Not assigned a FEMA number; not intended for flavor applications
Toxicology: Standard fragrance material safety profile; sensory testing and consumer exposure assessments recommended for specific applications. Not classified as a fragrance allergen requiring mandatory labeling under EU Regulation 1223/2009
References
Arctander, S. (1969). Perfume and flavor materials of natural origin. Elizabeth, NJ: Self-published.
Common Fragrance and Flavor Materials: Preparation, Properties and Uses. (2002). Weinheim: Wiley-VCH.
Pybus, D. H., & Sell, C. S. (Eds.). (1999). The chemistry of fragrances: From perfumer to consumer. Cambridge: Royal Society of Chemistry.
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Oxford: Blackwell Publishing.
Sell, C. S. (Ed.). (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Cambridge: Royal Society of Chemistry.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Weinheim: Wiley-VCH.