Methyl Ionone Gamma A Technical Ingredient Overview
🏭 Manufacturer — IFF (International Flavors & Fragrances); also produced by major fragrance houses including Givaudan, Firmenich, Symrise
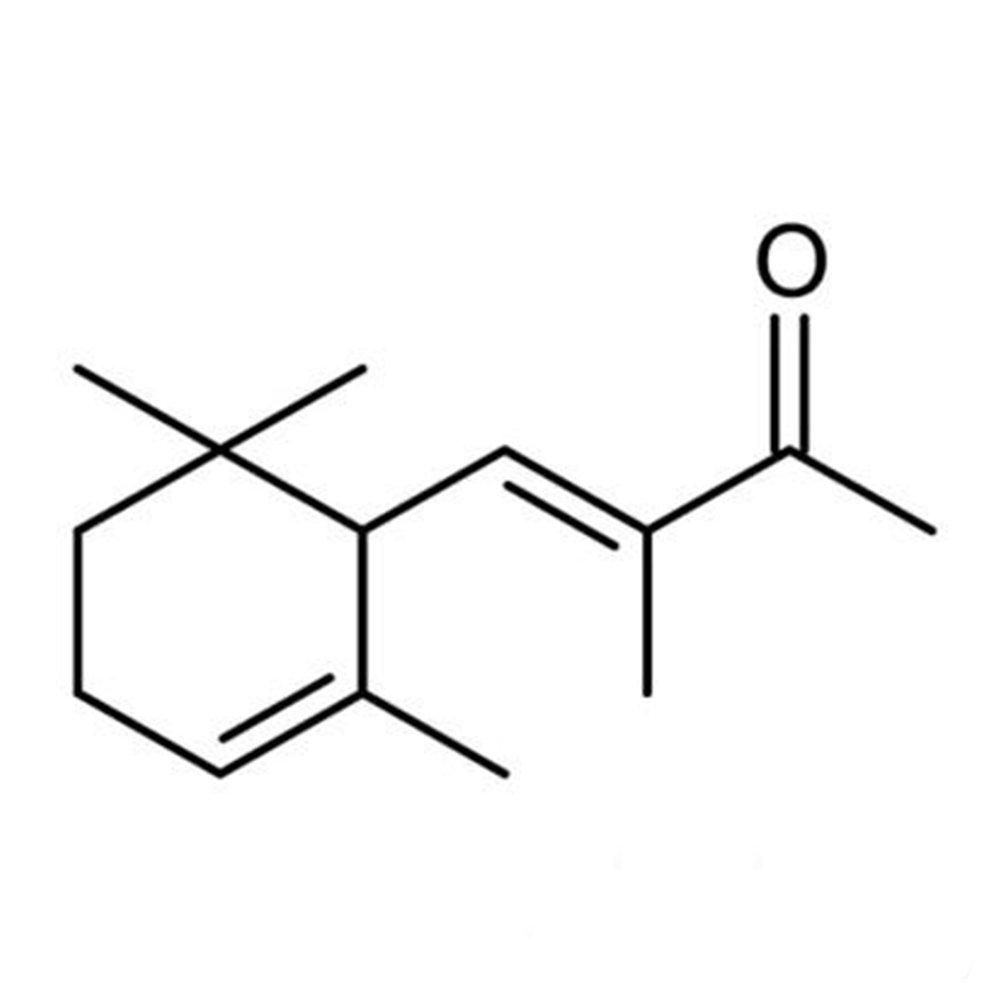
🔎 Chemical Name — Methyl ionone (mixture of isomers, predominantly α-isomethyl ionone)
🧪 Synonyms — Methylionone; Isoraldeine; Iralia; Methyl irisone; Ionone, methyl-; α-Isomethyl ionone (major component); Iraldeine gamma; Isoraldeine 95
📂 CAS Number — 1335-46-2 (mixture); Individual isomers: 127-42-4 (α-methylionone), 127-43-5 (β-methylionone), 127-51-5 (α-isomethyl ionone), 7779-30-8, 79-89-0, 1335-94-0
📘 FEMA Number — 2711 (methyl ionone mixture); 2714 (individual isomers)
⚖️ Molecular Weight — 206.33 g/mol (C₁₄H₂₂O)
📝 Odor Type — Floral-woody, violet
📈 Odor Strength — Medium to strong (varies by isomeric composition)
👃🏼 Odor Profile — Floral, velvety, violet-like with orris character; creamy, powdery top notes transitioning to woody, dry-fruity base; characteristic Parma violet and iris root facets with subtle leather and beeswax undertones
⚗️ Uses — Fine fragrance (floral, chypre, oriental compositions), orris and violet reconstructions, modifier and volume enhancer in woody-floral accords, luxury perfumery, cosmetic fragrances
🧴 Appearance — Colorless to pale straw-yellow, oily liquid
What is Methyl Ionone Gamma A?
Methyl ionone gamma A represents a specific commercial blend within the methyl ionone family, comprising multiple structural isomers with the molecular formula C₁₄H₂₂O. This material belongs to the ionone class of fragrance ingredients—cyclic terpenoid ketones originally derived from carotenoid degradation, though now produced exclusively through synthetic routes (Arctander, 1960; Sell, 2006).
The designation "gamma A" indicates a particular isomeric composition optimized for specific olfactory characteristics, with α-isomethyl ionone (CAS 127-51-5) typically constituting 60-70% of the mixture. This predominance of the α-isomethyl ionone isomer, characterized by its superior violet-orris character compared to other methylionone isomers, distinguishes gamma formulations from alpha or beta methylionone products.
Chemically, methylionones are characterized by a trimethylcyclohexenyl ring system attached to a pentenone or butenone side chain, with the specific position of double bonds and methyl group attachments determining the distinct olfactory properties of each isomer. Unlike simple ionones (C₁₃H₂₀O), methylionones possess an additional methyl substituent on the side chain, providing enhanced substantivity and modified olfactory profiles particularly valued in orris-type compositions.
The "A" suffix in commercial nomenclature typically denotes a premium or refined grade with controlled isomeric composition and superior olfactory performance characteristics compared to standard industrial methylionone mixtures.
Historical Background
The ionone family represents one of the earliest and most significant achievements in synthetic fragrance chemistry. β-Ionone was first synthesized by Ferdinand Tiemann and Paul Krüger in 1893, marking a milestone in the ability to recreate natural floral odors through synthetic means (Sell, 2006). This breakthrough enabled perfumers to access violet and iris notes without reliance on costly and inconsistent natural extracts.
The development of methylionone variants followed in the early 20th century as chemists explored structural modifications to the ionone scaffold. By introducing an additional methyl group to the side chain, researchers discovered compounds with enhanced tenacity, modified olfactory profiles, and superior performance in certain floral compositions—particularly in recreating the expensive and highly valued scent of orris root (Iris pallida, Iris germanica).
Key historical milestones:
1893: First synthesis of β-ionone by Tiemann and Krüger
Early 1900s: Development of methylionone variants through structural modifications of the ionone framework
Mid-20th century: Commercial production and optimization of isomeric mixtures for specific olfactory profiles
1960s-1970s: Recognition of α-isomethyl ionone as the most powerful and elegant isomer for orris reconstructions
Late 20th century: Establishment of standardized commercial blends (e.g., "Gamma," "Alpha Extra," "Coeur" designations) by major fragrance houses
The material gained particular prominence in luxury perfumery during the mid-to-late 20th century, becoming essential in violet-type compositions and serving as a cornerstone ingredient in orris reconstructions—significantly more economical than natural iris concrete or absolute, which require vast quantities of rhizomes and extended aging periods.
Contemporary methylionone gamma formulations represent refined versions of these early synthetics, with controlled isomeric ratios optimized through advanced separation and cyclization techniques.
Olfactory Profile
Scent Family
Methyl ionone gamma A belongs to the floral-woody family, specifically classified as a violet-orris type fragrance material with powdery-cosmetic undertones.
Main Descriptors
The olfactory character of methyl ionone gamma A presents a sophisticated, multifaceted profile that evolves significantly from initial impression through dry-down:
Primary facets:
Floral-violet: Characteristic Parma violet sweetness with refined elegance
Orris-root: Dry, powdery iris rhizome character with earthy-rooty undertones
Creamy-velvety: Smooth, luxurious texture particularly evident in top notes
Secondary facets:
Woody-dry: Cedar-like, dry-woody base notes
Fruity: Subtle dried-fruit nuances, occasionally described as plum-like
Powdery-cosmetic: Classic face-powder, lipstick-like associations
Tertiary facets:
Leather: Faint suede-like, almost animalic undertones at higher concentrations
Beeswax: Warm, waxy facets contributing to depth and naturalness
Earthy-rooty: Authentic rhizome character reminiscent of natural orris
The material's olfactory profile is notably cleaner and more refined than β-methylionone, which tends toward woody-cedarwood territories with less floral elegance. The predominance of α-isomethyl ionone in gamma formulations provides the characteristic smooth, velvety violet quality that made this isomer historically valuable.
Intensity
Methyl ionone gamma A demonstrates medium to strong olfactory impact, with intensity varying based on concentration and surrounding compositional elements. Individual isomers within the mixture exhibit significantly different odor thresholds—α-isomethyl ionone being notably more powerful than other methylionone isomers.
At typical usage levels (1-10% in compound), the material provides substantial presence without overwhelming delicate floral notes. Its intensity can be modulated through:
Concentration adjustment (effective range: 0.5-25% in fragrance concentrate)
Blending with woody-amber bases (which moderate violet intensity)
Combination with salicylates and terpene alcohols (which soften powdery character)
Tenacity
Exceptional persistence characterizes methyl ionone gamma A, with documented longevity exceeding 124 hours on perfumer's strips at standard evaluation concentrations (10% in ethanol). This remarkable substantivity derives from:
Relatively high molecular weight (206.33 g/mol)
Hydrophobic character (log Kow 4.84)
Low vapor pressure (0.005 mmHg at 20°C)
The material's tenacity makes it valuable as both a characterizing ingredient and a fixative component, particularly in:
Orris-violet reconstructions requiring sustained floral presence
Woody-floral bases demanding long-lasting bloom
Cosmetic fragrances where persistent powdery notes are desired
Volatility
Methyl ionone gamma A functions primarily in the heart to base note register, with initial diffusion providing immediate violet impact that evolves into sustained woody-powdery persistence. The material's volatility profile (boiling point 238-266°C at 101.3 kPa) positions it as a semi-volatile ingredient with:
Initial phase (0-30 minutes): Creamy-floral violet notes with orris character
Development phase (30 minutes - 6 hours): Evolution into woody-powdery territory with enhanced earthy-rooty facets
Dry-down phase (6+ hours): Sustained woody-powdery base with subtle leather and beeswax nuances
Fixative Role
While not classified as a traditional base-note fixative in the manner of musks or resins, methyl ionone gamma A contributes significant holding power and substantivity to fragrance compositions. Its fixative properties manifest through:
Stabilization of volatile florals: Extends longevity of ephemeral violet, rose, and jasmine notes
Bridging function: Links volatile citrus-floral top notes to heavier woody-ambery bases
Structural reinforcement: Provides backbone to floral-oriental and chypre compositions
Enhancement of other fixatives: Synergizes with woody materials (cedarwood, sandalwood, vetiver) and balsamic notes
Applications in Fine Fragrance
Methyl ionone gamma A occupies a distinguished position in contemporary luxury perfumery, serving both as a characterizing ingredient and as a sophisticated modifier. The material finds extensive application across multiple fragrance categories:
Violet and Orris Compositions
The material represents the cornerstone of modern violet-type perfumery, providing authentic Parma violet character while simultaneously delivering the dry, earthy, powdery facets associated with natural orris concrete. In these compositions, methyl ionone gamma A typically comprises 15-40% of the fragrance concentrate, often combined with:
α-Ionone and β-ionone: For additional floral complexity and vintage violet character
Orris butter or absolute (when budget permits): For naturalness and depth
Cassie absolute, mimosa: For honeyed-powdery floral effects
Iris pallida: When seeking premium natural reinforcement
Classic examples utilizing prominent methylionone notes include vintage violet fragrances and contemporary orris-centric compositions where the ingredient provides both top-note bloom and sustained dry-down presence.
Chypre and Floriental Structures
In chypre constructions, methyl ionone gamma A serves a dual role:
Floral enhancement: Rounds and softens oakmoss-patchouli bases
Powdery-dry modifier: Introduces cosmetic elegance bridging citrus-floral top notes and woody-mossy bases
The material pairs particularly effectively with:
Oakmoss products, tree moss: Creating classic floral-mossy chypres
Labdanum, benzoin: In ambery-oriental-chypre hybrids
Rose otto, geranium: For floral-chypre compositions
Bergamot, clary sage: Supporting herbaceous-aromatic dimensions
Typical usage: 5-15% of fragrance concentrate in chypre bases.
Woody-Leather Accords
The subtle leather and beeswax facets present in methyl ionone gamma A make it valuable in suede-type and woody-leather compositions, where it contributes:
Powdery-soft leather effects: As opposed to harsh, birch tar-type leather notes
Warmth and depth: In sandalwood-cedar-vetiver frameworks
Cosmetic elegance: Softening aggressive woody-amber bases
Particularly effective combinations include:
Vetiver oils (Haiti, Java): The material softens sharp, green, earthy edges
Cedarwood (Atlas, Virginian): Enhancing dry-woody character with floral polish
Sandalwood, Javanol: Creating creamy-woody effects with powdery overtones
Leather bases (isobutyl quinoline, birch tar): Providing soft, suede-like refinement
Salicylate-Floral Systems
Methyl ionone gamma A demonstrates remarkable synergy with benzyl salicylate and related compounds, creating voluminous, diffusive, floral-cosmetic effects particularly valued in:
Lily-type compositions: Where the material enhances waxy-green-floral effects
Ylang-ylang reconstructions: Adding powdery-creamy depth to indolic florals
Tuberose-narcissus bases: Introducing earthy-powdery facets
Cosmetic-aldehydic florals: Providing substantivity and orris refinement
The combination of methylionone + salicylates + musks represents a classic structural element in many prestige feminine fragrances, delivering the characteristic smooth, enveloping, diffusive quality associated with luxury floral perfumery.
Performance in Formula
Methyl ionone gamma A exhibits predictable and reliable performance characteristics in fragrance formulations:
Blending Behavior: The material demonstrates excellent compatibility with most perfumery ingredients, showing particular affinity for:
Woody materials (cedarwood, sandalwood, vetiver, patchouli)
Floral absolutes and essential oils (rose, jasmine, ylang-ylang, neroli)
Salicylates and benzoates
Synthetic musks (galaxolide, ambrettolide, macrocyclic musks)
Ambery bases and balsams
Some caution is advised when combining with highly aldehydic or very green notes, as the powdery-floral character may dampen freshness if used at excessive concentrations.
Typical Usage Levels:
Orris-violet focused compositions: 15-40%
Floral modifiers in complex accords: 3-15%
Woody-floral bases: 5-20%
Chypre and oriental structures: 3-12%
Functional fragrances: 2-8%
Stability Considerations: Methyl ionone gamma A demonstrates excellent stability in alcoholic solutions and across most product matrices:
Alcoholic perfumes: Excellent stability, no discoloration
Emulsions (creams, lotions): Stable across typical pH ranges (4-8)
Soaps: Some potential for reaction at very high pH (>11); use with caution in hot-process soaps
Detergents and cleaners: Generally stable though some odor attenuation may occur
Light sensitivity: Minimal photodegradation; suitable for products in clear glass packaging
Oxidation resistance: Good stability; no significant autoxidation issues under normal storage conditions
Solubility:
Excellent in ethanol, diethyl phthalate, isopropyl myristate
Soluble in 8 volumes of 70% ethanol
Limited water solubility (as expected for lipophilic materials)
Industrial & Technical Uses
Beyond fine fragrance applications, methyl ionone gamma A serves functional purposes across multiple industries:
Cosmetics and Personal Care
Face powders and compacts: Reinforces cosmetic-powdery character
Lipsticks and lip products: Contributes signature cosmetic-floral notes
Creams and lotions: Provides elegant floral-powdery dry-down
Shampoos and hair care: Imparts long-lasting floral-orris notes with good substantivity on hair
Deodorants: Creates sophisticated floral-fresh effects with tenacity
Functional Fragrances
Fabric softeners: Delivers persistent floral-powdery notes on textiles
Laundry detergents: Provides floral-fresh effects with reasonable performance through wash cycle
Air fresheners: Contributes elegant floral notes with good diffusion characteristics
Household cleaners: When floral-cosmetic signatures are desired (though usage may be limited by cost)
Flavor Applications
Minor use in flavor formulations (FEMA 2711 approved), particularly in:
Floral-fruity candy flavors
Violet and orris mimetics for confectionery
Specialty fruit flavors requiring violet-berry notes
Note: Flavor usage is limited compared to perfumery applications due to the distinct, somewhat soapy taste profile that is less universally appreciated in food contexts.
Regulatory & Safety Overview
IFRA Status
Methyl ionone (mixed isomers) is subject to quantitative restrictions under IFRA Standards (Amendment 49, revised 2020; confirmed in Amendment 51, 2023). The material has specific maximum concentration limits based on dermal sensitization considerations:
Maximum acceptable concentrations in finished consumer products:
The above limits apply to methyl ionone isomers used individually or in combination.
IFRA Standards Library: https://ifrafragrance.org/standards/IFRA_STD_063.pdf
EU Cosmetics Regulation
Under EU Regulation (EC) No 1223/2009 (Annex III), α-isomethyl ionone (the predominant component of methyl ionone gamma A) is listed among the 26 declarable fragrance allergens:
Labeling requirements:
Leave-on products: Must be declared if present ≥ 0.001% (10 ppm)
Rinse-off products: Must be declared if present ≥ 0.01% (100 ppm)
Labeling format: List "Alpha-Isomethyl Ionone" in the INCI ingredient declaration
This allergen status applies specifically to α-isomethyl ionone (CAS 127-51-5), which constitutes approximately 60-70% of methyl ionone gamma A formulations. Manufacturers using this material in cosmetic products must ensure proper allergen declaration on product labels when thresholds are exceeded.
FEMA Status
Methyl ionone mixture is approved for flavor use:
FEMA Number: 2711
Status: GRAS (Generally Recognized As Safe) when used in accordance with good manufacturing practices
Individual isomers may have separate FEMA designations (e.g., FEMA 2712, 2714)
Flavor applications remain minor relative to perfumery usage.
Toxicology
The safety profile of methyl ionone has been extensively evaluated by the Research Institute for Fragrance Materials (RIFM) and the Expert Panel for Fragrance Safety:
Acute Toxicity:
Oral LD₅₀ (rat): >5,000 mg/kg (RIFM, 1973; Lalko et al., 2007)
Dermal LD₅₀: Expected to be >5,000 mg/kg based on structural analogues
Classification: Low acute toxicity
Dermal Sensitization:
Recognized dermal sensitizer at certain exposure levels
IFRA restrictions derived from quantitative risk assessment (QRA2 methodology)
Human Repeated Insult Patch Test (HRIPT) data indicate sensitization potential at higher concentrations
No Observed Effect Level (NOEL) established through guinea pig maximization tests
Skin Irritation:
Mild to non-irritating at use concentrations
Potential for irritation at concentrations significantly above recommended levels
Eye Irritation:
Mild to moderate eye irritant potential (undiluted material)
Appropriate caution advised in products with potential eye contact
Phototoxicity/Photoallergenicity:
No evidence of phototoxic or photoallergenic potential
Systemic Toxicity:
Low systemic toxicity based on repeated dose studies
No evidence of reproductive or developmental toxicity at relevant exposure levels
No mutagenic or genotoxic concerns identified
Environmental Profile:
Log Kow: 4.84 (indicates moderate bioaccumulation potential)
Biodegradability: Expected to be readily biodegradable based on structural characteristics
Aquatic toxicity: Moderate toxicity to aquatic organisms; requires appropriate risk management in rinse-off applications
Natural Occurrence
Unlike the parent ionones (α, β, γ-ionone), which occur naturally as carotenoid degradation products in various plants, methylionones are not definitively confirmed natural constituents of essential oils or plant materials. While some historical literature suggests possible natural occurrence, this remains disputed and unconfirmed by modern analytical techniques (Sell, 2006).
The structural similarity to naturally occurring ionones suggests that methylionones could theoretically form through analogous biosynthetic pathways involving carotenoid breakdown and subsequent methylation reactions. However, if present in nature, concentrations would be trace and unlikely to be economically extractable.
Confirmed natural ionone sources (for context):
Rosa damascena (Bulgarian rose oil): Contains β-damascone and β-ionone
Viola odorata (Violet): Contains ionones contributing to characteristic scent
Various Boronia species: Minor ionone constituents
Osmanthus species: β-ionone and related compounds
The absence of economically viable natural sources has led to exclusive reliance on synthetic production for all commercial methylionone materials.
Production Methods
Methyl ionone gamma A, like all commercial methylionones, is produced through synthetic routes involving multi-step processes analogous to ionone synthesis. Production involves:
Step 1: Pseudocompound Formation
The synthesis begins with preparation of a linear "pseudomethylionone" precursor through aldol-type condensation:
Route A: From Citral
Reactants: Citral (or 6-methylcitral) + methyl ethyl ketone
Catalyst: Alkaline catalysts (NaOH, KOH) for n-methylpseudoionone; quaternary ammonium bases for isomethylpseudoionone
Product: Mixture of n-methylpseudoionone and isomethylpseudoionone as geometric isomers (cis/trans)
Route B: From Dehydrolinalool (Modern industrial preference)
Reactants: Dehydrolinalool + appropriate α,β-unsaturated ketone or enol ether of methyl ethyl ketone
Advantage: Direct access from synthetic terpene alcohol; avoids citral dependence
Product: Predominantly isomethylpseudoionone when using appropriate catalysis
The ratio of linear (n-methyl) to branched (isomethyl) pseudocompound isomers depends critically on catalyst choice and reaction conditions, directly influencing the final methylionone isomer distribution.
Step 2: Cyclization to Methylionones
The pseudomethylionone undergoes acid-catalyzed cyclization to form the trimethylcyclohexenyl ring system:
Cyclization catalysts:
Concentrated sulfuric acid (85-98%): Produces primarily α-methylionone with some β-isomer
Phosphoric acid (85%): Yields β-methylionone in higher proportion
Boron trifluoride etherate (BF₃·Et₂O): In DMF solvent, produces γ-methylionone alongside α and β isomers
Lewis acids (AlCl₃, ZnCl₂): Alternative catalysts with varying selectivities
The cyclization mechanism involves protonation of the terminal double bond, electrophilic attack on the trisubstituted double bond, and subsequent ring closure with simultaneous formation of the conjugated enone system.
Step 3: Isomer Separation and Blending
Following cyclization, the crude product contains multiple methylionone isomers (α, β, γ forms, each potentially as multiple geometric and optical isomers). Industrial processing involves:
Fractional distillation:
High-efficiency columns separate isomers based on boiling point differences
α-methylionone: bp ~97°C @ 0.35 kPa
β-methylionone: bp ~102°C @ 0.35 kPa
α-isomethylionone: bp ~130-131°C @ 1.3 kPa
β-isomethylionone: bp ~94°C @ 0.4 kPa
Controlled blending: Commercial products like "Methyl Ionone Gamma A" represent carefully formulated blends of specific isomers (primarily α-isomethyl ionone at 60-70%, with controlled proportions of complementary isomers) to achieve desired olfactory profiles. Different suppliers and trade names (Iralia®, Isoraldeine, etc.) reflect varying isomeric compositions optimized for specific applications.
Modern Industrial Variations
Contemporary production may incorporate:
Continuous flow reactors for pseudocompound formation
Advanced separation technologies (preparative chromatography for high-purity specialty grades)
Enantioselective synthesis methods (for research and premium applications, though commercial methylionones are racemic mixtures)
Green chemistry approaches utilizing heterogeneous catalysis and reduced solvent systems
Major Producers
Global production of methylionones (all commercial grades) occurs at major fragrance houses and specialized aroma chemical manufacturers, including IFF, Givaudan, Firmenich, Symrise, and dedicated chemical companies supplying the fragrance industry.
References
Arctander, S. (1960). Perfume and flavor materials of natural origin. Elizabeth, NJ: Arctander.
European Commission. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (Annex III: List of substances which cosmetic products must not contain except subject to the restrictions laid down). Official Journal of the European Union.
International Fragrance Association. (2020). IFRA Standard: Methyl ionone, mixed isomers (Amendment 49). Retrieved from https://ifrafragrance.org/standards/IFRA_STD_063.pdf
International Fragrance Association. (2023). IFRA Standards—51st Amendment. Retrieved from https://ifrafragrance.org/standards-library
Lalko, J., Lapczynski, A., McGinty, D., Bhatia, S. P., Letizia, C. S., & Api, A. M. (2007). Fragrance material review on methyl ionone (mixture of isomers). Food and Chemical Toxicology, 45(Suppl. 1), S235-S240. https://doi.org/10.1016/j.fct.2007.09.054
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Oxford: Blackwell Publishing.
Sell, C. S. (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Cambridge: Royal Society of Chemistry.
Sigma-Aldrich. (2025). Methyl ionone (mixture of isomers) [Product specifications]. Retrieved from https://www.sigmaaldrich.com
U.S. Food and Drug Administration. (2024). FEMA GRAS Assessment: Methyl ionone (FEMA 2711). Flavor and Extract Manufacturers Association.