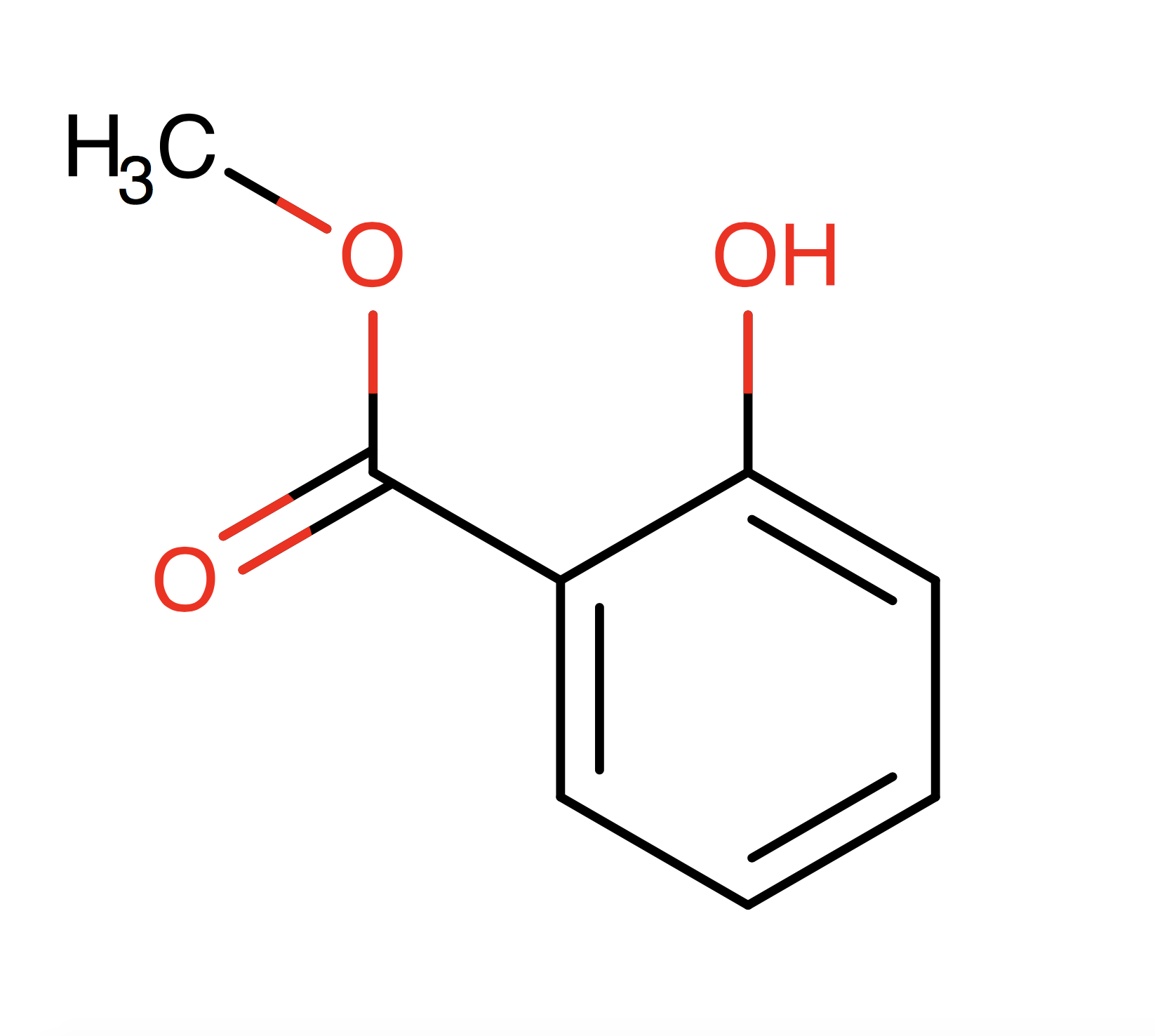
Methyl Salicylate - Technical Ingredient Overview
🔎 Chemical Name — Methyl 2-hydroxybenzoate
🧪 Synonyms — Oil of wintergreen, Wintergreen oil, Betula oil, Sweet birch oil, Gaultheria oil, Salicylic acid methyl ester, O-Hydroxybenzoic acid methyl ester, 2-Carbomethoxyphenol
📂 CAS Number — 119-36-8
📘 FEMA Number — 2745
⚖️ Molecular Weight — 152.15 g/mol
📝 Odor Type — Phenolic, sweet-aromatic
📈 Odor Strength — Very strong, powerful odorant with low perceptibility threshold (0.05-0.10 mg%)
👃🏼 Odor Profile — Intensely sweet-aromatic with characteristic wintergreen note, creamy-fruity topnote, phenolic-medicinal character, sweet-woody dryout. North American perspective emphasizes refreshing, minty qualities; European perspective emphasizes medicinal, liniment character
⚗️ Uses — Perfumery modifier in blossom fragrances, fougère compositions, and forest notes; mild antiseptic in oral hygiene products; flavoring agent in confectionery, beverages, and tobacco; pharmaceutical applications
🧴 Appearance — Colorless to pale yellow, pinkish, or yellowish liquid with sweet wintergreen aroma
What is Methyl Salicylate?
Methyl salicylate (C₈H₈O₃) is an organic aromatic ester derived from salicylic acid and methanol. It represents one of the most culturally distinctive fragrance materials in perfumery, with perception and acceptance varying dramatically between North America and other global markets. The compound exists both as a natural constituent of several plant species—most notably wintergreen (Gaultheria procumbens) and sweet birch (Betula lenta)—and as a synthetically produced material through esterification processes (Bauer et al., 2001).
As a phenolic ester, methyl salicylate belongs to the salicylate family of fragrance materials, which includes commercially important compounds such as benzyl salicylate, hexyl salicylate, and isoamyl salicylate. These materials share structural similarities but differ significantly in their olfactory profiles and applications. Natural methyl salicylate comprises over 95-99% of wintergreen and birch essential oils, with only trace amounts of other volatile constituents including α-pinene, myrcene, δ-3-carene, limonene, and cadinene (Arctander, 1960).
The material's historical significance extends beyond perfumery into pharmaceutical applications, where its close chemical relationship to salicylic acid (the precursor to aspirin) has established its therapeutic value for analgesic and anti-inflammatory purposes.
Historical Background
Methyl salicylate was first isolated in 1843 by French chemist Auguste André Thomas Cahours (1813-1891) from the wintergreen plant Gaultheria procumbens. Cahours successfully identified the compound as an ester of salicylic acid and methanol, establishing the foundation for understanding this material's chemical nature and potential applications (Bauer et al., 2001).
The commercial production of methyl salicylate initially relied entirely on natural extraction through steam distillation of wintergreen leaves and sweet birch twigs. The production process required maceration of plant material in warm water to enable enzymatic action on glycosides within the plant tissue, which liberated methyl salicylate. This enzymatic conversion was critical, as the compound does not exist in free form within the living plant but rather as a glycosidic precursor. The distillation process typically required overnight maceration followed by approximately two-thirds of a day for complete distillation (Arctander, 1960).
By the mid-20th century, production of natural wintergreen oil had become concentrated in the eastern United States, particularly Pennsylvania, Connecticut, Tennessee, and the Carolinas around the Southern Appalachians. In northern Pennsylvania, wintergreen oil production served as a supplementary winter income source for farmers. The industry established two commercial grades—Northern and Southern—with the Northern grade commanding premium pricing due to its purportedly superior fragrance character, despite being chemically identical (Arctander, 1960).
The shift toward synthetic production occurred gradually as chemical manufacturing capabilities advanced and demand increased. Synthetic methyl salicylate is produced through direct esterification of salicylic acid with methanol in the presence of sulfuric acid catalyst. This process proved far more economical and scalable than natural extraction, leading to synthetic material largely replacing natural wintergreen and birch oils in industrial applications by the latter half of the 20th century. Today, natural wintergreen oil production has declined to the point where genuine material may be measured in hundreds of kilograms rather than tons annually (Arctander, 1960).
The cultural history of methyl salicylate reveals fascinating geographic preferences. In North America, wintergreen flavor became deeply embedded in popular culture through traditional uses in root beer, birch beer, sarsaparilla, chewing gum, and oral hygiene products. This created strong positive associations that persist in American markets. Conversely, European markets generally rejected wintergreen-based products, with the aroma primarily associated with medicinal liniments and muscle rubs rather than pleasant flavoring (Bauer et al., 2001).
Olfactory Profile
Scent Family
Phenolic-aromatic with strong salicylate character. Belongs to the phenolic ester family alongside benzyl salicylate and other aromatic salicylates.
Main Descriptors
The olfactory profile of methyl salicylate exhibits notable cultural interpretation differences. North American perfumers and consumers describe the material as refreshing, sweet-minty, and wintergreen-like with pleasant fruity-creamy aspects. The topnote presents creamy-fruity nuances, while the heart develops intensely sweet-aromatic character with phenolic warmth. The dryout phase reveals sweet-woody notes with potential tarlike aspects in poorly distilled natural oils (Arctander, 1960).
European perfumers typically characterize the same material with emphasis on its medicinal, liniment-like qualities—reminiscent of muscle rubs and pharmaceutical preparations. This perception stems from widespread European use of methyl salicylate in topical analgesic products rather than flavoring applications. The sweet phenolic character reads as distinctly pharmaceutical rather than confectionery in these markets (Bauer et al., 2001).
High-quality methyl salicylate displays a peculiar creamy-fruity topnote with remarkable sweetness and aromatic complexity. The phenolic warmth provides depth without harshness when used appropriately. Natural wintergreen and birch oils exhibit subtle complexity from trace constituents that synthetic material lacks, though these differences require expert evaluation to detect.
Intensity
Methyl salicylate ranks among the most powerful odorants in perfumery. The minimum perceptible concentration ranges from 0.05 to 0.10 mg% in finished products, indicating exceptional olfactory potency. Suggested use levels in flavored goods typically range from 0.20 to 0.50 mg%, though oral hygiene products may contain concentrations of 300-700 mg% (Arctander, 1960). Trace amounts in fragrance compositions exert significant impact on the overall olfactory impression.
Tenacity
The material demonstrates excellent substantivity and fixative properties. Sweet-woody dryout notes persist for extended periods, contributing to the base note development of fragrances. The phenolic character provides longevity typical of aromatic esters with ortho-substituted hydroxyl groups.
Volatility
Methyl salicylate exhibits moderate volatility characteristics, functioning primarily as a middle-to-base note material. Boiling point: 223.3°C; vapor pressure: 0.0975 mmHg at 20°C. The material's relatively low volatility compared to other aromatic esters contributes to its fixative properties and lasting impact in fragrance compositions (Bauer et al., 2001).
Fixative Role
Functions effectively as a mild fixative in floral and herbaceous compositions. The material enhances longevity of more volatile components while contributing characteristic sweet-aromatic warmth. Blends particularly well with other phenolic materials, coumarinic notes, and floral absolutes where it adds natural depth and fixation.
Applications in Fine Fragrance
Methyl salicylate finds specialized application as a modifier and accent material in specific fragrance families. Its primary uses include ylang-ylang, tuberose, narcissus, lily, and gardenia reconstructions where trace amounts contribute authentic natural depth and complexity. The material adds characteristic warmth and sweet-phenolic nuances to white floral compositions (Arctander, 1960).
Fougère fragrances represent the material's classical perfumery application. Historically recommended for fougère bases, methyl salicylate has been identified as a natural constituent in fern stems, lending authenticity to these compositions. The characteristic fougère note can be achieved through combinations of oakmoss, coumarin, and amyl salicylate, with methyl salicylate used in trace amounts for added complexity and natural character (Arctander, 1960).
Forest and herbaceous compositions benefit from judicious methyl salicylate additions, particularly in pine fragrances, chypres, aldehydic bases, and masculine cologne formulations. The material introduces fresh topnotes while providing warm, rich body and natural roundness at higher concentrations.
Successful accord partnerships include bergamot oil, coumarin and derivatives, heliotropine, ionones, isoborneol acetate, geranium oil, labdanum, lavender and lavandin oils, synthetic musks, various salicylates, rose absolute, oakmoss products, patchouli oil, sandalwood oil, sage clary oil, vanillin derivatives, vetiver oil, and ylang-ylang oil (Arctander, 1960).
Performance in Formula
Methyl salicylate demonstrates excellent blending behavior with both natural and synthetic fragrance materials. The material's phenolic-aromatic character integrates smoothly into complex compositions without dominating when used at appropriate concentrations.
In blends, methyl salicylate exhibits synergistic relationships with anetholic, vanillic, mentholated, and terpenoid components. The material enhances perceived freshness in citrus-forward compositions while adding substantive body to florals. Its interaction with coumarin produces characteristic fougère effects that remain central to this fragrance family.
The material's solubility profile facilitates easy incorporation into both alcoholic and oil-based fragrance systems. Soluble in alcohol, ether, acetic acid, and most common organic solvents; insoluble in water. Specific gravity at 25°C: 1.174 g/mL. Refractive index (n₂₀/D): 1.536-1.539 (Bauer et al., 2001).
Industrial & Technical Uses
Beyond fine fragrance applications, methyl salicylate serves multiple industrial functions. The material acts as a high-temperature heat carrier in textile dyeing operations. In microscopy and histology, methyl salicylate's high refractive index (1.536) makes it valuable for tissue clearing and specimen preparation, particularly in immunofluorescence analysis where it rapidly renders samples transparent while preserving structure.
The compound functions as an effective rust remover and degreasing agent for machinery, proving particularly effective against seawater corrosion. Industrial solvent applications include use in adhesives, glues, inks, paints, and polymer processing. Agricultural applications exploit methyl salicylate as an insect attractant and plant defense signaling compound (Bauer et al., 2001).
Regulatory & Safety Overview
IFRA Status
Methyl salicylate does not appear in the IFRA 51st Amendment restricted or prohibited standards index, indicating no specific IFRA-imposed restrictions at current publication. However, individual manufacturers should verify current regulatory status as standards evolve. Access current IFRA Standards at: https://ifrafragrance.org/standards-library
EU Cosmetics Regulation
Classified under GHS with hazard statements H302 (harmful if swallowed), H315 (causes skin irritation), H319 (causes serious eye irritation), H335 (may cause respiratory irritation). Classified as Acute Tox. 4 Oral, Eye Dam. 1, Repr. 2, Skin Sens. 1B, and Aquatic Chronic 3 under CLP Regulation.
FEMA Status
Approved as FEMA 2745 with GRAS (Generally Recognized As Safe) status for food flavoring applications. ADI (Acceptable Daily Intake): 0-0.5 mg/kg body weight. Authorized for use in strawberry, vanilla, grape, and other fruit flavor formulations for foods and beverages.
Toxicology
Methyl salicylate presents significant toxicity concerns, particularly for children. Oral LD₅₀ values: mouse 1110 mg/kg, rat 887 mg/kg, rabbit 1300 mg/kg. Human oral LD₅₀: child 228 mg/kg, adult 506 mg/kg. A single teaspoon (5 mL) contains approximately 6 g salicylate—equivalent to nearly twenty 300 mg aspirin tablets. The lowest published lethal dose: 101 mg/kg body weight in adults; as little as 4 mL has proven fatal to small children (Bauer et al., 2001).
Topical absorption through intact skin can produce systemic salicylate toxicity, including CNS respiratory stimulation, metabolic disturbances, and in severe cases, acute lung injury, seizures, cerebral edema, and death. Salicylate sensitivity manifests as allergy-like symptoms or asthma in susceptible individuals. The material is contraindicated during pregnancy and lactation due to transdermal absorption. Individuals taking anticoagulant medications should avoid exposure due to potentiation of bleeding risk.
References
Arctander, S. (1960). Perfume and flavor materials of natural origin. Published by the author.
Bauer, K., Garbe, D., & Surburg, H. (2001). Common fragrance and flavor materials: Preparation, properties and uses(4th ed.). Wiley-VCH.
Cahours, A. A. T. (1843). [First isolation of methyl salicylate from Gaultheria procumbens]. Annales de Chimie et de Physique.
International Fragrance Association (IFRA). (2023). IFRA Standards—51st Amendment. Retrieved from https://ifrafragrance.org/standards-library
Pybus, D. H., & Sell, C. S. (Eds.). (1999). The chemistry of fragrances: From perfumer to consumer. Royal Society of Chemistry.
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Blackwell Publishing.