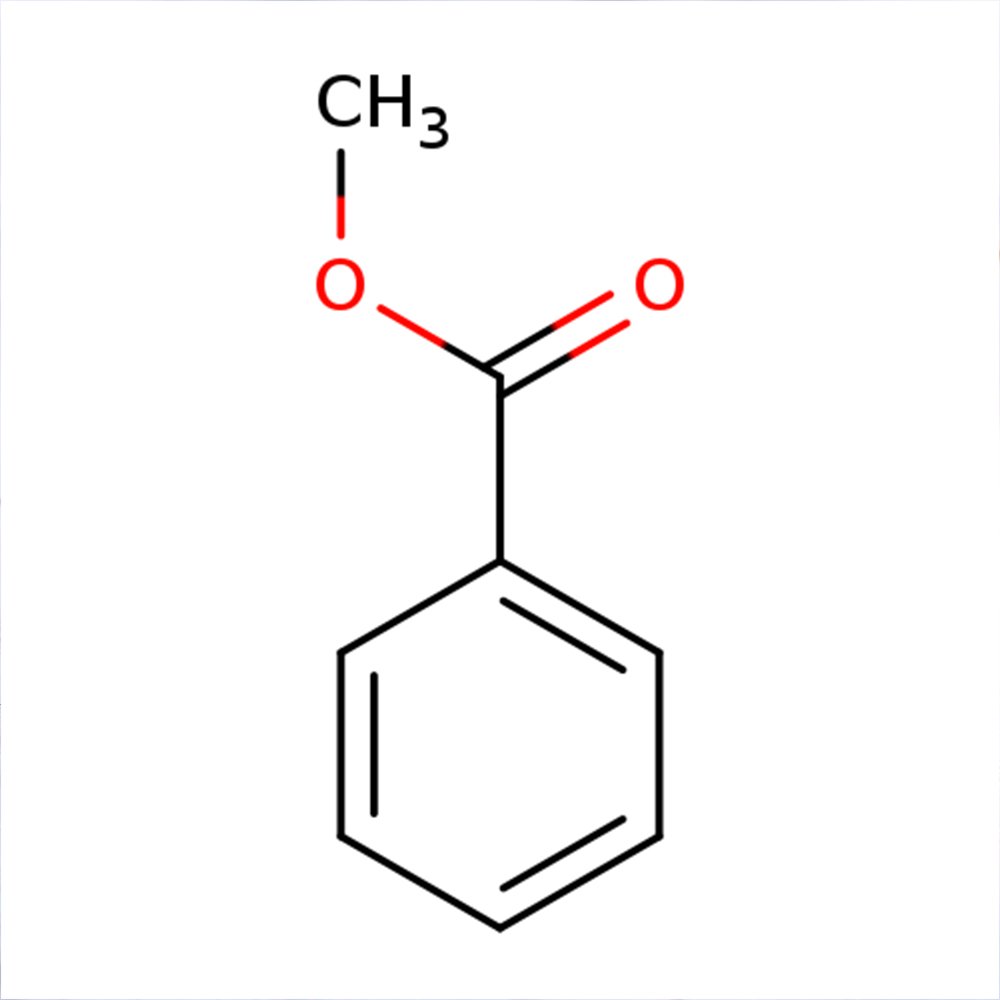
Methyl Benzoate - Technical Ingredient Overview
🔎 Chemical Name — Methyl benzoate; Benzoic acid methyl ester
🧪 Synonyms — Oil of Niobe, Niobe Oil, Clorius, Methyl benzenecarboxylate, Essence of Niobe
📂 CAS Number — 93-58-3
📘 FEMA Number — 2683
⚖️ Molecular Weight — 136.15 g/mol
📈 Molecular Formula — C₈H₈O₂ (C₆H₅COOCH₃)
📝 Odor Type — Aromatic ester, narcotic-floral
📈 Odor Strength — Strong (8/10)
👃🏼 Odor Profile — Narcotic, phenolic, sweet-floral with fruity undertones reminiscent of ylang-ylang and tuberose; sharp, medicinal, bittersweet character with notes of feijoa fruit, cherry pit, wintergreen, and camphor; dry-fruity with powdery facets
⚗️ Uses — Perfume bases (ylang-ylang, tuberose, narcissus types), flavor compositions (cherry, vanilla, tropical fruits), industrial solvent, insect attractant (orchid bees), forensic marker (cocaine degradation product)
🧴 Appearance — Colorless to slightly yellow transparent liquid; poorly soluble in water, miscible with organic solvents
What is Methyl Benzoate?
Methyl benzoate is an aromatic ester formed through the condensation of benzoic acid and methanol. As a simple benzoate ester with the chemical formula C₆H₅COOCH₃, it belongs to the aromatic ester chemical family and serves as a fundamental building block in perfumery and flavor chemistry (Surburg & Panten, 2006).
This compound exhibits a characteristic narcotic-floral odor profile with strong phenolic overtones, making it particularly valuable in white floral compositions. While naturally occurring in trace amounts in essential oils such as ylang-ylang, tuberose, clove, and narcissus, commercial applications rely almost exclusively on synthetic production due to economic viability and consistency (Arctander, 1969).
Methyl benzoate's dual nature as both a perfumery material and industrial intermediate gives it unique significance. In the polymer industry, it emerges as a large-volume by-product during the manufacture of polyethylene terephthalate (PET/Terylene), making high-quality material readily available for fragrance applications at competitive costs (Rowe, 2005).
Historical Background
The synthesis of aromatic esters through esterification reactions was established in the mid-19th century as organic chemistry developed systematic methodologies. While specific documentation of methyl benzoate's first synthesis is limited in historical records, the compound became commercially relevant to perfumery in the late 1800s as synthetic chemistry expanded the perfumer's palette beyond natural extracts alone.
By the early 20th century, methyl benzoate was recognized as a conventional ingredient in classic perfume types such as Peau d'Espagne and became standard in ylang-ylang reconstitutions (Arctander, 1969). Its industrial-scale availability increased significantly with the development of the petrochemical industry and polymer manufacturing, particularly from the 1950s onward when it emerged as a by-product in PET production.
The material's adoption in perfumery was driven by both its olfactive qualities—offering a cost-effective alternative to rare natural extracts—and its technical versatility as a solvent and fixative in various formulation contexts.
Olfactory Profile
Scent Family
Aromatic Ester — Falls within the benzoate ester subfamily with narcotic-floral characteristics
Main Descriptors
Primary: Narcotic, phenolic, medicinal, sweet-floral
Secondary: Fruity (feijoa, cherry pit), powdery, balsamic, sharp
Tertiary: Wintergreen-like, camphoraceous, bittersweet
The scent is characterized by its duality: a sharp, phenolic-medicinal top note transitions into a sweet, heavy floral heart reminiscent of ylang-ylang and tuberose. In dilution, subtle fruity-balsamic nuances emerge, though the material retains a persistent medicinal undertone that distinguishes it from softer esters like ethyl benzoate.
Intensity
Strong (8/10) — High olfactory impact with low odor threshold (~110 ppb). Detectable even at trace concentrations, making it effective at minimal dosage levels (Burdock, 2010).
Tenacity
Moderate to Good — Functions across top-to-middle notes with reasonable substantivity. While more volatile than benzyl benzoate, it demonstrates better longevity than simple aliphatic esters.
Volatility
Top-to-Middle Note — Evaporation rate positions it as a volatile modifier in floral compositions, though it extends into the heart of a fragrance with sufficient persistence to influence mid-development.
Fixative Role
While not a classic base note fixative, methyl benzoate contributes structural stability to narcotic floral accords. It anchors volatile fruity-floral notes and adds weight to ephemeral compositions, particularly in ylang-ylang and tuberose bases where it reinforces natural ester profiles present in the absolutes.
Applications in Fine Fragrance
Methyl benzoate serves specialized roles in perfume construction:
Narcotic White Florals: Essential in ylang-ylang, tuberose, and narcissus bases, where its phenolic-sweet character reinforces natural extraction profiles
Vintage Floral Reconstructions: Conventional ingredient in classic Peau d'Espagne and Fougère types
Spicy-Floral Transitions: Provides medicinal-balsamic contrast in compositions featuring clove, carnation, or cinnamon
Leather Accords: Contributes phenolic sharpness and dry-fruity nuances to labdanum and birch tar compositions
Typical Use Level: 0.1–1.5% in fine fragrance compounds, though higher concentrations may be employed in industrial perfumes and masking formulations.
Performance in Formula
Methyl benzoate exhibits predictable blending behavior with excellent compatibility across most perfumery materials. It pairs particularly well with:
Benzyl acetate: Enhances floral softness
Cinnamic alcohol: Amplifies balsamic warmth
Methyl anthranilate: Creates grape-neroli effects
Cresyl ethers/esters: Reinforces phenolic sharpness
Its high diffusion rate and strong initial impact make it valuable for lift in heavy floral bases, though care must be taken to avoid overwhelming delicate compositions with its sharp medicinal facet.
Industrial & Technical Uses
Solvent Applications: Cellulose esters, cellulose ethers, resins, rubber
Polyester Dyeing: Auxiliary agent for hydrophobic polyester fiber dyeing
Entomology: Standard attractant for male orchid bees (Euglossini) used in field studies
Forensic Science: Volatile marker for cocaine degradation; used in training drug detection dogs
Chemical Synthesis: Precursor for benzaldehyde and benzophenone derivatives
Regulatory & Safety Overview
IFRA Status
No restriction under IFRA Amendment 51 (2023). Not listed among restricted materials or prohibited substances. Can be used without specific concentration limits in all product categories (IFRA, 2023).
FEMA Status
FEMA #2683 — Recognized as GRAS (Generally Recognized As Safe) for use in food flavoring applications. Maximum usage: ~61 mg/kg in food products; ADI: 5 mg/kg body weight (Adams et al., 2005).
EU Cosmetics Regulation
Not a declarable allergen under EU Regulation 1223/2009. Not among the 26 fragrance allergens requiring labeling. Complies with EU Regulation 1334/2008 for use in flavorings.
Safety Profile
Acute Oral Toxicity (LD₅₀): 3.4 g/kg (rats) — classified as low acute toxicity
GHS Classification: Acute Tox. 4 (Oral), Repr. 2
Hazard Statements: H302 (harmful if swallowed), H361d (suspected of damaging the unborn child)
Handling: Store away from heat/ignition sources. Use in well-ventilated areas. Avoid prolonged skin contact.
Note: While generally considered safe at normal usage levels, methyl benzoate should be handled following good manufacturing practices and appropriate safety protocols.
References
Adams, T. B., Cohen, S. M., Doull, J., Feron, V. J., Goodman, J. I., Marnett, L. J., ... & Wagner, B. M. (2005). The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food and Chemical Toxicology, 43(8), 1207-1240.
Arctander, S. (1969). Perfume and flavor chemicals (aroma chemicals). Published by author.
Burdock, G. A. (2010). Fenaroli's handbook of flavor ingredients (6th ed.). CRC Press.
International Fragrance Association. (2023). IFRA Standards: 51st Amendment. https://ifrafragrance.org/standards-library
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Blackwell Publishing.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Wiley-VCH.