Macrolide Supra Technical Ingredient Overview
🏭 Manufacturer — Symrise
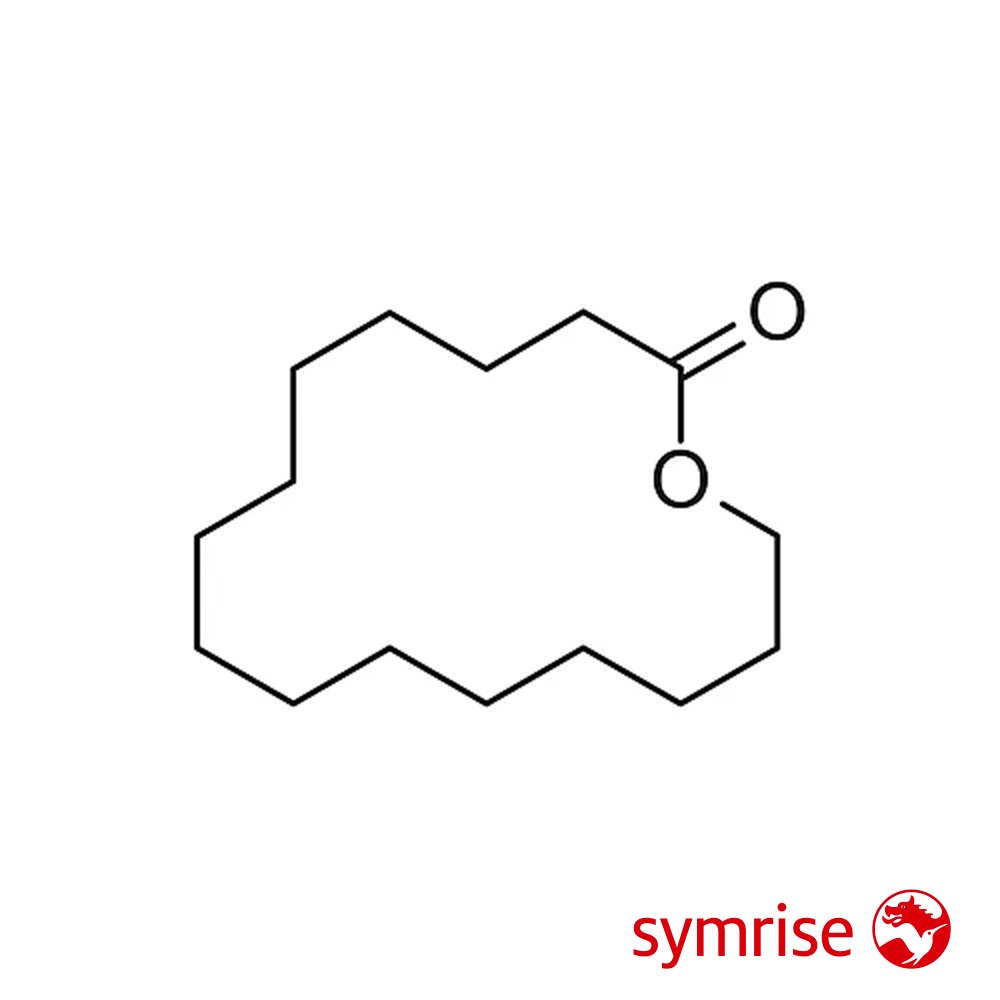
🔎 Chemical Name — Oxacyclohexadecan-2-one (1-Oxa-2-cyclohexadecanone)
🧪 Synonyms — 15-Pentadecanolide, Cyclopentadecanolide, ω-Pentadecalactone, Exaltolide, Pentalide, Thibetolide, Muskalactone, Muskolactone, 15-Hydroxypentadecanoic acid lactone, 2-Pentadecalone, Pentadecan-15-olide
📂 CAS Number — 106-02-5
📘 FEMA Number — 2840
⚖️ Molecular Weight — 240.38 g/mol
📝 Odor Type — Macrocyclic Musk, Lactone
📈 Odor Strength — Medium to Strong (Odor threshold: 1-4 ppb in air; 2.1 ng/L reported by Rowe, 2005)
👃🏼 Odor Profile — Delicate, sweet, musky, powdery, warm animal-like with subtle floral-fruity nuances reminiscent of ambrette seed. Slightly pastoral and herbal with angelica root character. Clean, elegant, and extremely tenacious with outstanding uniformity.
⚗️ Uses — Fixative and base note enhancer in fine fragrance; musk base in floral, woody, fruity, and chypre accords; functional fragrance in fabric care, personal care products, and air fresheners. Also employed in flavor applications (FEMA 2840) for vanilla, creamy, and licorice notes.
🧴 Appearance — White crystalline solid or colorless to pale yellow viscous liquid above melting point (34-38°C). Forms colorless crystals upon cooling. Solid at room temperature but easily melted for formulation.
What is Macrolide Supra?
Macrolide Supra is a synthetic macrocyclic lactone musk belonging to the family of 15-membered ring macrolides. Chemically identified as oxacyclohexadecan-2-one or 15-pentadecanolide, it is characterized by a large lactone ring containing 15 carbon atoms with one oxygen atom incorporated into the cyclic structure. This molecular architecture is responsible for its distinctive musky olfactory profile and exceptional substantivity.
Macrolide Supra represents a class of fragrance ingredients known as macrocyclic musks, which emerged as environmentally safer and olfactorily sophisticated alternatives to earlier nitro musks and polycyclic musks. The compound mimics the structure of naturally occurring musk compounds but is produced synthetically for commercial use, ensuring consistent quality, purity, and sustainability.
As a member of the lactone family, Macrolide Supra shares structural similarities with naturally occurring macrolides found in angelica root oil and ambrette seed oil. Its synthesis allows perfumers to access the elegant, clean musk character previously available only from expensive natural extracts or animal-derived musks, now rendered obsolete for ethical and regulatory reasons.
Historical Background
The discovery and development of 15-pentadecanolide represents a pivotal moment in the history of synthetic perfumery and the evolution of modern musk chemistry.
Discovery in Natural Sources (1927)
15-Pentadecanolide was first identified in 1927 by German chemist Max Kerschbaum of Haarmann & Reimer, who isolated the compound from Angelica root oil (Angelica archangelica L., syn. Archangelica officinalis Hoffm.) (Rowe, 2005). This discovery revealed that the distinctive musk-like character detectable in aged angelica root essential oil was attributable to the presence of macrocyclic lactones, particularly 15-pentadecanolide.
Kerschbaum's work demonstrated that the compound occurred naturally in small quantities—modern analytical studies have confirmed that 15-pentadecanolide accounts for approximately 0.87-14.9% of the macrolide fraction in angelica root oil, with concentrations increasing as volatile monoterpene hydrocarbons evaporate during storage and aging of the roots (Rowe, 2005; Kraft & Tochtermann, 1994).
Commercial Introduction as Exaltolide (1927-1930s)
Almost simultaneously with Kerschbaum's discovery, the Swiss fragrance house Firmenich introduced the synthetic version of 15-pentadecanolide to the perfumery industry under the trade name Exaltolide® in 1927. At the time of its introduction, Exaltolide was extraordinarily expensive, priced at an exorbitant 100,000 CHF per kilogram, making it one of the most costly perfumery ingredients ever commercialized (Rowe, 2005).
Initially, Firmenich produced Exaltolide through Baeyer-Villiger oxidation of cyclopentadecanone (Exaltone®), a related macrocyclic ketone. This synthetic route, while effective, was complex and costly, contributing to the prohibitive pricing.
Evolution of Synthetic Processes (1930s-1960s)
The high cost of macrocyclic musks drove intensive research into more efficient synthetic methodologies. A major breakthrough came from E.W. Spanagel and W.H. Carothers at DuPont in the 1930s, who developed a polycondensation process that circumvented the traditional high-dilution requirements for macrocyclization (Rowe, 2005). Their method involved:
Polycondensation of 15-hydroxypentadecanoic acid at 180-250°C in the presence of Lewis acid catalysts
Vacuum distillation of the monomeric 15-pentadecanolide at 270°C/1 Torr
This innovation reduced the price by a factor of ten, enabling ton-scale production and broader commercial adoption of the material.
Modern Industrial Synthesis (1960s-Present)
The real revolution in macrocycle synthesis began in the 1960s following the work of Günther Wilke at the Max Planck Institute for Coal Research, who pioneered the cyclooligomerization of butadiene to produce cyclododecanone on an industrial scale (now manufactured at >100,000 tons/year) (Rowe, 2005). This abundant industrial intermediate became the starting point for modern macrolide synthesis.
The current industrial process, developed in the 1970s-1980s, involves:
Radical addition of allyl alcohol to cyclododecanone
Acid-catalyzed cyclization to form a bicyclic enol ether intermediate
Oxidation with hydrogen peroxide to generate a hydroperoxide
Fragmentation in the presence of cupric acetate and iron sulfate to yield 11/12-pentadecen-15-olides (commercialized as Habanolide® and Globalide®)
Catalytic hydrogenation with Raney nickel to produce 15-pentadecanolide
This route enabled economical large-scale production, bringing the price down to approximately 60 CHF per kilogramby the 2000s (Rowe, 2005).
Marketing as Macrolide Supra by Symrise
Symrise (formerly Haarmann & Reimer) introduced Macrolide Supra as their premium-quality version of 15-pentadecanolide, marketed as a refined, cost-effective alternative to other brands. The "Supra" designation indicates superior quality specifications, emphasizing purity, olfactory elegance, and batch-to-batch consistency.
Today, multiple manufacturers produce 15-pentadecanolide under various trade names including Cyclopentadecanolide(Symrise), Thibetolide (Givaudan), Pentalide (Soda Aromatics/Bedoukian), and Exaltolide (Firmenich).
Olfactory Profile
Scent Family
Macrocyclic Musk, Lactone
Macrolide Supra belongs to the family of macrocyclic musks, characterized by large ring lactones (≥12 atoms) that impart persistent, diffusive musk notes without the harsh or animalic character of polycyclic musks.
Main Descriptors
Primary: Musky, powdery, soft animal-like
Secondary: Floral (reminiscent of ambrette seed), fruity (pear-like, peach), creamy, sweet
Tertiary: Pastoral, herbal (angelica root character), balsamic, vanilla-like, slightly woody
Character: Clean, elegant, extremely tenacious, uniform, delicate, cosmetic
The odor is often described as mimicking natural skin scent, lending a "clean musk" or "white musk" quality that enhances the wearability of perfumes on skin.
Intensity
Medium to Strong. Macrolide Supra has an exceptionally low odor threshold (1-4 ppb in air), making it one of the most potent musk molecules. Despite this high impact, the scent is perceived as soft and non-overpowering, contributing to its reputation as an elegant musk.
Tenacity
Extremely High. Macrolide Supra is renowned for its outstanding substantivity and longevity, providing exceptional fixative properties to fragrance compositions. It exhibits very low volatility (vapor pressure: 0.0003 hPa at 20°C; 0.08 Pa), allowing it to persist on skin and textiles for extended periods—often days to weeks in fabric applications.
Volatility
Low to Very Low (Base Note)
Classification: Heart-to-Base Note
Evaporation Rate: Slow
Boiling Point: 137°C at 2 mmHg (~280°C at atmospheric pressure)
Vapor Pressure: 0.0003 hPa at 20°C
Macrolide Supra's low volatility makes it an effective fixative, reducing the evaporation rate of more volatile top and middle notes while contributing its own persistent musk character to the dry-down phase of a fragrance.
Fixative Role
Macrolide Supra functions as both a physical fixative (reducing volatility through molecular weight and low vapor pressure) and an olfactory fixative (modifying and rounding the overall bouquet). Unlike simple physical fixatives, it actively modulates scent perception, creating smooth transitions between notes and amplifying diffusion when applied to skin. This property is sometimes referred to as its "synergistic effect on the bouquet."
Applications in Fine Fragrance
Macrolide Supra is a cornerstone ingredient in modern perfumery, valued for its versatility, elegance, and ability to enhance virtually any fragrance family.
Role in Fragrance Compositions
Musk Base: Provides the foundational musk character in both masculine and feminine fragrances
Fixative: Extends longevity and improves dry-down performance
Skin Enhancer: Mimics natural skin scent, creating intimate, sensual effects
Roundness & Depth: Adds smoothness, warmth, and three-dimensionality to compositions
Diffusion Booster: Enhances projection and sillage, particularly on skin
Pairing Behavior with Other Ingredients
Macrolide Supra blends exceptionally well with:
Other Musks: Combines with Galaxolide, Helvetolide, Ambrettolide, Muscenone for multi-faceted musk accords
Woody Alcohols: Enhances sandalwood derivatives, Javanol, Sandalore
Floral Isolates: Harmonizes with Hedione, Iso E Super, methyl ionones
Vanilla & Gourmand Notes: Complements ethyl maltol, vanillin, coumarin
Recommended Dosage:
Fine Fragrance: 0.1–3%
Functional Fragrance (Fabric Care, Air Care): 1–5%
Personal Care (Soap, Lotion, Shampoo): 0.2–1%
Performance in Formula
Behavior in Blends and Mixtures
Macrolide Supra exhibits excellent stability in various formulation matrices:
Alcoholic Solutions: Highly soluble in ethanol, propylene glycol, dipropylene glycol (DPG), and isopropyl myristate (IPM). Often pre-diluted in DPG (10%, 50%) for easier handling, as the material is solid at room temperature.
Aqueous Systems: Poorly soluble in water; requires solubilizers or emulsifiers for aqueous formulations.
Functional Products: Shows good stability in shampoos, body lotions, concentrated detergent powders, and AP roll-ons. Poor stability in bleach formulations.
Soap & Detergent: Excellent retention in bar soaps and fabric care products, providing long-lasting freshness to textiles.
Impact on Overall Composition
Synergistic Effects: Macrolide Supra enhances the diffusion and perception of co-formulants, creating a "halo effect" that amplifies the overall bouquet.
Smoothing Agent: Softens harsh or sharp notes, providing seamless transitions between fragrance phases.
Skin Affinity: Improves perfume performance on skin by mimicking natural skin lipids and enhancing "skin acceptance and radiance."
Non-Discoloring: Does not impart color to formulations, maintaining the visual integrity of products.
Formulation Considerations
Melting Point: 34-38°C (solid at room temperature). Requires gentle heating (water bath) or dilution in solvents for incorporation into cold formulations.
Dosage Control: Due to high odor impact and low threshold, precision in dosing is critical. Overdosage is the most frequent abuse, leading to overpowering muskiness.
pH Stability: Stable across a wide pH range, suitable for acidic (citric acid cleaners) and alkaline (detergent powders) applications.
Light & Heat Sensitivity: Exhibits good photostability and thermostability under typical storage conditions.
Industrial & Technical Uses
Beyond fine fragrance, Macrolide Supra finds extensive application in functional and industrial formulations:
Fragrance Applications
Fabric Care: Detergents, fabric softeners, dryer sheets for long-lasting freshness
Personal Care: Shampoos, conditioners, body washes, lotions, deodorants
Home Care: Air fresheners, surface cleaners, multipurpose cleaners
Fine Fragrance: Eau de parfum, eau de toilette, cologne
Cosmetics: Powders, creams, makeup formulations
Flavor Applications (FEMA 2840)
Macrolide Supra is approved for use as a flavoring agent (FEMA 2840) in food and beverages:
Flavor Profile: Vanilla bean, powdery heliotropine, creamy, licorice, musk
Applications: Alcoholic beverages, dentifrice flavors, confectionery, dairy products
Typical Dosage: 0.02–1.50 ppm in finished consumer products (noting that dentifrice flavors are formulated at ~100× concentration)
Non-Fragrance Industrial Uses
Polymer Chemistry: Monomer for the synthesis of poly(pentadecanolide) (PPDL) via enzyme-catalyzed ring-opening polymerization (Novozyme 435 catalyst)
Pharmaceutical Applications: Used in the development of directly compressed prolonged-release tablets for slightly soluble drugs (e.g., ketoprofen)
Chemical Synthesis: Starting material for terminally-branched iso-fatty acids (iso-C15-C17)
Biochemical Research: Investigated as a potential selective inhibitor of rat liver cyclic AMP-dependent protein kinase (cAK) with IC50 = 20 μM
Regulatory & Safety Overview
IFRA Status
Not Restricted under current IFRA Standards (Amendment 51, 2023).
15-Pentadecanolide (CAS 106-02-5) does not appear on the IFRA Standards list, indicating that it is not subject to specific use-level restrictions across the 12 product categories. This reflects the favorable safety profile of the material based on comprehensive toxicological and dermatological assessments.
For the most current regulatory status, consult the official IFRA Standards Library: https://ifrafragrance.org/standards-library
EU Cosmetics Regulation
Not Listed among the 26 declarable fragrance allergens in Annex III of EU Cosmetics Regulation (EC) No 1223/2009.
Macrolide Supra is not required to be declared on ingredient lists when present in cosmetic products, as it is not recognized as a significant sensitizer or allergen at typical use concentrations.
EC Number: 203-354-6
FEMA Status
FEMA Number: 2840
FEMA Designation: Generally Recognized as Safe (GRAS) for use as a flavoring substance.
Macrolide Supra is approved by the Flavor and Extract Manufacturers Association (FEMA) for use in food and beverages. The material meets purity specifications established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
Flavis Number: 10.004
Council of Europe Number: 181
Toxicology
Macrolide Supra has been extensively evaluated for safety and demonstrates a favorable toxicological profile:
Acute Oral Toxicity (Rats): LD50 > 5,000 mg/kg (Levenstein, 1974)
Acute Dermal Toxicity (Rabbits): LD50 > 5,000 mg/kg (Levenstein, 1974)
Skin Irritation: Non-irritating or minimally irritating at typical use levels
Skin Sensitization: Not considered a significant sensitizer; patch testing shows low irritation potential
Eye Irritation: May cause mild eye irritation; avoid direct contact
Mutagenicity/Genotoxicity: No evidence of mutagenic or genotoxic effects in standard assays
Reproductive Toxicity: No evidence of reproductive or developmental toxicity at relevant exposure levels
GHS Classification: H410 (Very toxic to aquatic life with long-lasting effects)
Hazard Class: Aquatic Acute 1, Aquatic Chronic 2
Biodegradability
Readily Biodegradable. Macrocyclic musks, including 15-pentadecanolide, are readily biodegradable under aerobic conditions, distinguishing them favorably from polycyclic musks (PCMs) that are persistent in the environment (Rowe, 2005).
Biodegradation: >60% mineralization within 28 days (OECD 301 test)
Environmental Fate: Does not bioaccumulate significantly in aquatic organisms
Regulatory Preference: Increasingly favored over polycyclic musks due to superior environmental profile
Aquatic Toxicity
Low to Moderate. While classified as H410 (very toxic to aquatic life with long-lasting effects) under GHS labeling, the material's ready biodegradability mitigates long-term environmental impact. Standard disposal procedures should be followed to minimize unnecessary environmental exposure.
Environmental & Sustainability Considerations
Environmental Profile
Macrolide Supra benefits from a favorable environmental profile compared to earlier generations of synthetic musks:
Readily Biodegradable: Degrades rapidly in aerobic wastewater treatment, reducing accumulation in aquatic ecosystems
Low Bioaccumulation: Does not accumulate significantly in fish or marine organisms, unlike polycyclic musks
Non-Persistent: Does not persist in sediments or soil; breaks down efficiently under natural conditions
Reduced Ecotoxicity: Lower aquatic toxicity compared to nitro musks and certain polycyclic musks
Sustainability Considerations
Synthetic Origin: Production does not depend on animal sources (e.g., musk deer) or endangered plant species, ensuring ethical sourcing
Renewable Feedstocks: Modern synthesis routes utilize petroleum-derived cyclododecanone, though research into bio-based alternatives is ongoing
High Performance Efficiency: Low usage levels (0.1-3%) due to high odor impact, reducing overall material consumption per formulation
Regulatory Compliance: Meets stringent environmental regulations in the EU, US, and other major markets
Market Trends
Shift Away from Polycyclic Musks: Driven by environmental concerns, fragrance houses increasingly favor macrocyclic musks like Macrolide Supra
"Clean Musk" Formulations: Growing demand for "green," "clean label," and allergen-free musk solutions positions Macrolide Supra as a preferred choice
Sustainable Perfumery: Aligns with industry initiatives promoting biodegradable, non-bioaccumulative fragrance ingredients
References
Kraft, P., & Tochtermann, W. (1994). Ring enlargement of cyclodecanone by a chiral building block: Synthesis and olfactory properties of (12R)-(+)-12-methyl-13-tridecanolide. Liebigs Annalen der Chemie, 1994, 1161-1164.
Levenstein, I. (1974). Acute toxicity data for cyclopentadecanolide. Unpublished toxicology report.
PubChem. (2024). Pentadecanolide (CID 235414). National Center for Biotechnology Information. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/235414
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Blackwell Publishing. Chapter 7: Aroma Chemicals IV: Musks, pp. 147-167.
Sigma-Aldrich. (2024). Pentadecanolide product specification sheet (CAS 106-02-5). Retrieved from https://www.sigmaaldrich.com
Symrise. (2024). Macrolide Supra technical data sheet. Symrise AG Ingredient Finder.