Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Jasmonal A (Amyl Cinnamal)
Synthetic Ingredient for Perfumery
This essence combines a very mild, oily-herbaceous, and subtly floral aroma, reminiscent of natural blooms like Jasmine, Gardenia, and Tuberose, with sweet, floral, fruity, tropical, and green nuances, finished with a powdery touch. Extensively used in perfumery and soap fragrances, it introduces a Jasmine-like floralness and blends excellently, enhancing fragrance fixation due to its tenacity.
Synthetic Ingredient for Perfumery
This essence combines a very mild, oily-herbaceous, and subtly floral aroma, reminiscent of natural blooms like Jasmine, Gardenia, and Tuberose, with sweet, floral, fruity, tropical, and green nuances, finished with a powdery touch. Extensively used in perfumery and soap fragrances, it introduces a Jasmine-like floralness and blends excellently, enhancing fragrance fixation due to its tenacity.
Synthetic Ingredient for Perfumery
This essence combines a very mild, oily-herbaceous, and subtly floral aroma, reminiscent of natural blooms like Jasmine, Gardenia, and Tuberose, with sweet, floral, fruity, tropical, and green nuances, finished with a powdery touch. Extensively used in perfumery and soap fragrances, it introduces a Jasmine-like floralness and blends excellently, enhancing fragrance fixation due to its tenacity.
Synthetic Ingredient Overview
🏭 Manufacturer — IFF
📚Synonyms: Jasmonal A, α-Amylcinnamaldehyde, 2-Benzylideneoctanal
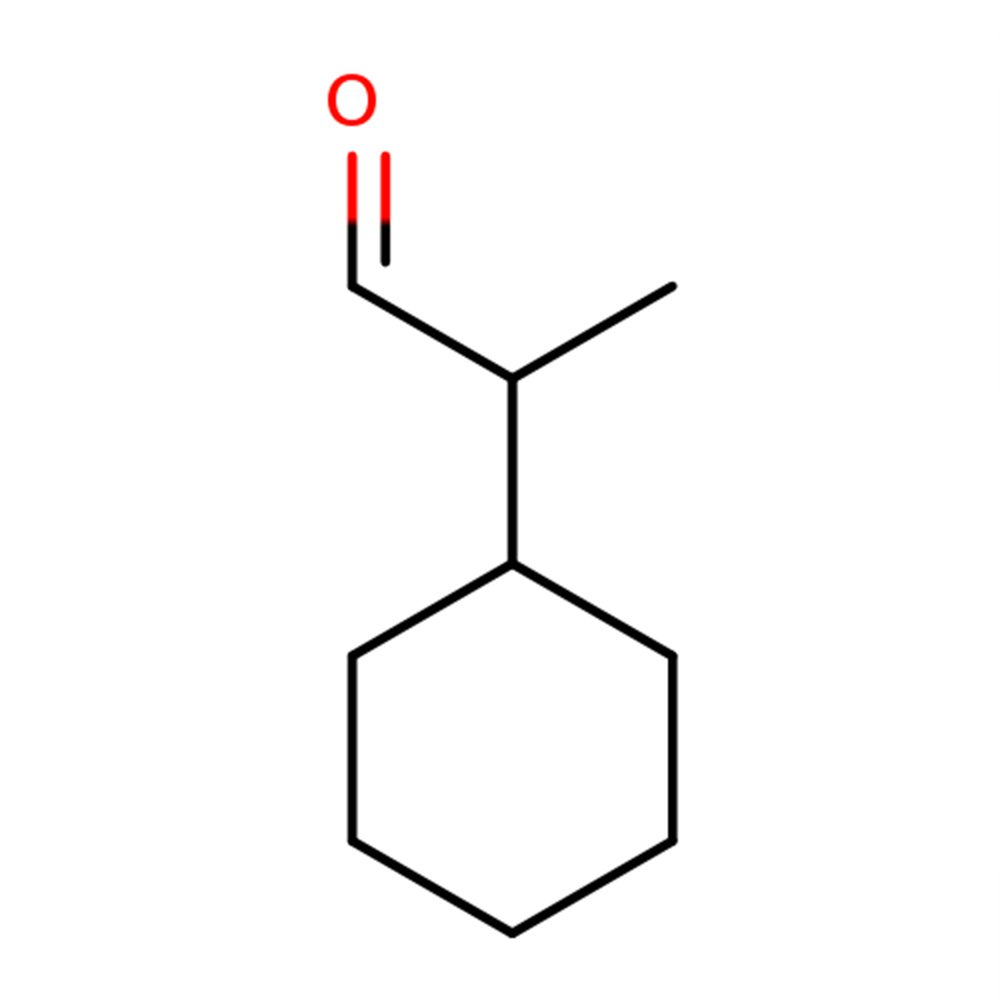
👓 Chemical Formula: C₁₄H₂₀O
🔎 Chemical name — (2Z)-2-benzylideneheptanal
📂 CAS N° — 122-40-7
📘 FEMA —2677
📕 EINECS: 204-541-5
⚖️ MW — 202.29 g/mol
📝 Odor type — Floral → Jasmin
📈 Odor Strength — Medium
👃🏼 Odor Profile —Very mild oily-herbaceous and somewhat floral odor, reminiscent of many types of natural flowers, but mostly of Jasmin, Gardenia and Tuberose (Arctander) \\ Sweet, floral, fruity, tropical and green with a powdery nuance (Mosciano, Gerard P&F 22, No. 6, 41, (1997))
👅Flavor Profile — Tropical, waxy, floral, rosy and honey-like with a fruity nuance and body (Mosciano, Gerard P&F 22, No. 6, 41, (1997))
⚗️ Uses — Used very extensively in perfumes, including soap perfumes. Introduces Jasmin-like floral-ness when accompanied by more volatile chemicals of floral character. Blends excellently and assists in fixation of the fragrance (ACA is quite tenacious)
👁️ Appearance: Pale yellow to yellow liquid
Historical Context
Alpha-Amyl Cinnamic Aldehyde, commonly known as Jasmonal A, holds historical significance as one of the earliest cost-effective synthetic substitutes for jasmine absolute. Developed and introduced in the mid-20th century, it emerged during a period when the fragrance industry was actively seeking stable and affordable materials to reproduce the complex profiles of expensive natural absolutes such as jasmine, rose, and orange blossom (Sell, 2019).
Rather than mimicking jasmine’s heady indolic character, Jasmonal A contributed warm, sweet, and diffusive floral tones with a creamy, ambery facet—making it ideal for reinforcing jasmine bouquets and complementing ylang-ylang and tuberose. It played a key role in the first wave of jasmine reconstructions, often used alongside benzyl acetate, benzyl alcohol, methyl anthranilate, and cis-jasmone to build jasmine-type accords in a more economical and industrially viable way (Arctander, 1960).
Its impact extended beyond fine perfumery. Due to its high stability, low cost, and strong substantivity, Alpha-Amyl Cinnamic Aldehyde became a staple in functional perfumery—especially in soaps, shampoos, lotions, and detergents—where it imparted a soft floral warmth that could survive alkaline or surfactant-rich environments. Its enduring presence in legacy functional bases underlines its practical value throughout the second half of the 20th century.
As with many older materials, its use has become more selective in recent decades due to IFRA restrictions and its inclusion among the 26 EU declarable allergens, which has led many perfumers to reformulate or minimize its dosage. Nevertheless, it remains an important reference molecule in jasmine fantasy florals and a symbol of the transition from natural-based perfumery to synthetic-floral innovation.
Discovery and First Synthesis
Alpha-Amyl Cinnamic Aldehyde is synthesized via aldol condensation between heptanal (C7 aldehyde) and benzaldehyde, a classical organic reaction forming a conjugated α,β-unsaturated aldehyde. This structural class—similar to cinnamaldehydes—contributes significantly to its warm, balsamic, floral-spicy profile.
Commercialized under various trade names, including Jasmonal A, it became an important tool in fine fragrance formulation in the mid-1900s, as part of the broader movement toward synthetic jasmine substitutes alongside materials like methyl dihydrojasmonate, cis-jasmone, and benzyl acetate (Arctander, 1960).
First Uses
Originally employed to enrich and reinforce jasmine accords, Alpha-Amyl Cinnamic Aldehyde quickly found a place in a wide range of floral and oriental fragrances. Its unique balance of floralcy and warmth made it a useful modifier in:
Ylang-ylang and tuberose blends
Spicy florals and soft orientals
Fruity-floral compositions, where it lends creamy diffusion and depth
It has also been used in functional perfumery (e.g., soaps and detergents) due to its tenacity and low volatility.
Production Method
Synthesis:
Prepared via base-catalyzed aldol condensation between benzaldehyde and heptanal
The resulting compound is 2-benzylideneoctanal, classified as an α,β-unsaturated aldehyde
The product is then purified by fractional distillation
Commercial Grades:
Often sold in high-purity grades under trade names such as Jasmonal A, Amylcinnamaldehyde, or Cinnamic Aldehyde, Alpha-Amyl
Olfactory Profile
Odor Description: Floral, warm, jasminic, sweet, slightly spicy, balsamic
Volatility: Mid to base note
Tenacity: Strong
Character: Floral-jasmine with woody and ambery nuances
Threshold: Perceptible at low ppm levels
“Intensely floral, heavy and sweet, with a definite resemblance to jasmine absolute, though with a balsamic and amylic background.” — Arctander, 1960
Applications in Perfumery
Alpha-Amyl Cinnamic Aldehyde is used in:
Jasmine and white floral bouquets
Oriental, spicy, and ambery blends
Creamy fruity-florals and ylang-ylang-based perfumes
Functional perfumery (e.g., soaps and shampoos) for its tenacity
Used in combination with Hedione, Benzyl acetate, Indole, and cis-Jasmone for naturalistic jasmine reconstitution
It also finds a role in aromatic accords where a subtle animalic or ambery nuance is needed.
IFRA Status and Safety Considerations
IFRA: Restricted
Maximum level in leave-on skin products: up to 0.15% depending on product category
Restricted due to its potential as a skin sensitizer
Allergen Declaration:
Listed among the 26 EU declarable allergens under Regulation No. 1223/2009
Must be labeled when exceeding 0.001% (leave-on) or 0.01% (rinse-off)
Hazard Statements:
H317: May cause allergic skin reaction
H411: Toxic to aquatic life with long-lasting effects
Flash Point: ~113 °C
Environmental & Sustainability Aspects
Readily biodegradable under standard conditions
Classified as aquatic toxic; proper wastewater treatment and dilution controls are required
Produced from widely available petrochemical intermediates (benzaldehyde and heptanal)
Appendix – References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
Burdock, G. A. (2010). Fenaroli’s Handbook of Flavor Ingredients (6th ed.). CRC Press.
Sell, C. S. (2019). The Chemistry of Fragrances (3rd ed.). Cambridge: Royal Society of Chemistry.
ECHA. (2024). Alpha-Amyl Cinnamic Aldehyde – Substance Information. European Chemicals Agency. Retrieved from https://echa.europa.eu
IFRA. (2023). IFRA Standards – Amendment 51. International Fragrance Association. Retrieved from https://ifrafragrance.org