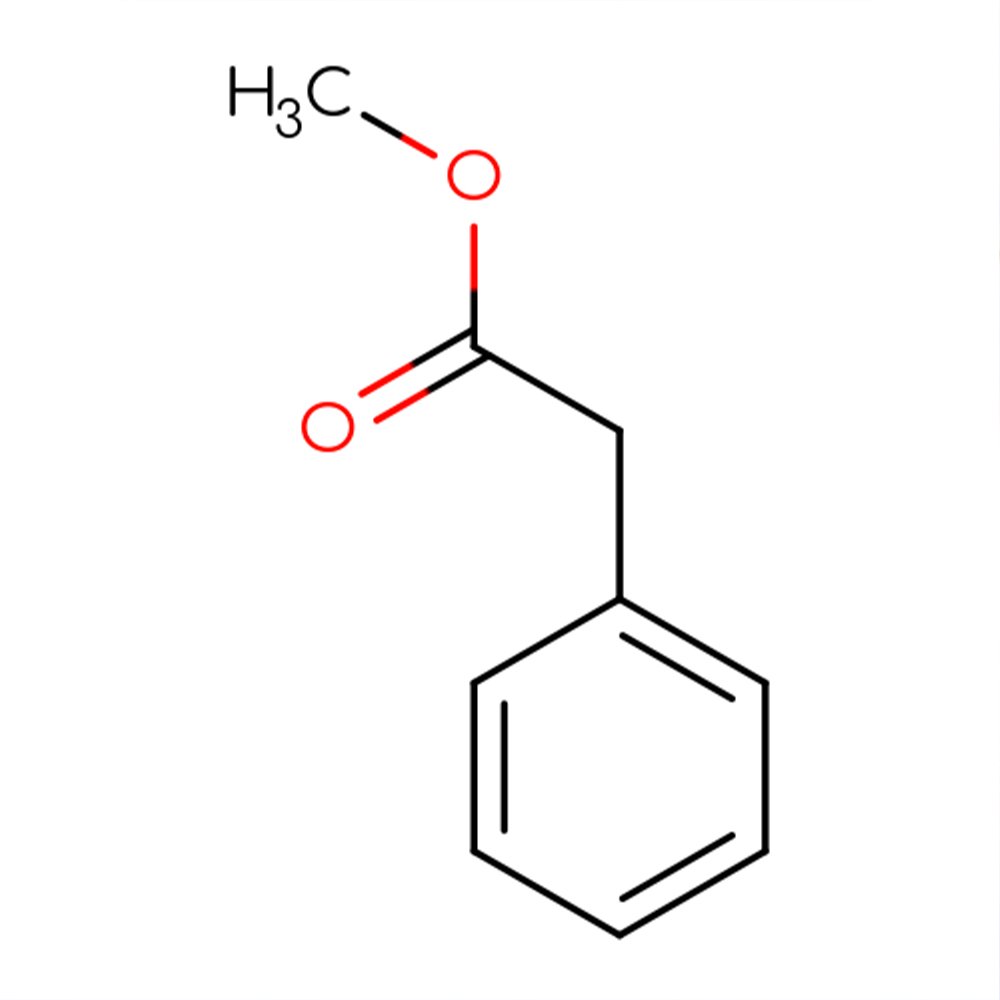
Technical Ingredient Overview
🔎 Chemical Name — 2-Methylpropyl phenylacetate; Benzeneacetic acid, 2-methylpropyl ester
🧪 Synonyms — Phenylacetic acid isobutyl ester, Isobutyl α-toluate, α-Toluic acid isobutyl ester, 2-Methylpropyl 2-phenylacetate, Eglantine, Iphaneine
📂 CAS Number — 102-13-6
📘 FEMA Number — 2210
💧 EC Number — 203-007-9
🔢 Flavis Number — 09.788
⚖️ Molecular Weight — 192.25 g/mol
🧬 Molecular Formula — C₁₂H₁₆O₂
📝 Odor Type — Sweet floral-gourmand with honey-cocoa tonalities
📈 Odor Strength — Medium to strong; recommended evaluation at one to ten percent dilution in perfumer's alcohol
👃🏼 Odor Profile — Sweet cocoa-honey dominant with floral undertones, waxy-spicy nuances, and subtle fruity facets. Presents a rounded, confectionery-like character with musk-like depth. At higher concentrations, the cocoa note becomes more pronounced with a balsamic warmth reminiscent of fine chocolate and honeycomb (Sigma-Aldrich, 2024; ChemicalBook, 2024).
⚗️ Uses — Flavor and fragrance agent for honey-cocoa accords in fine and functional perfumery; flavoring agent in confectionery, chocolate, and honey flavor systems; modifier in floral-oriental compositions
🧴 Appearance — Colorless to pale yellow clear liquid with characteristic sweet odor; refractive index (n²⁰/D) approximately 1.487; density approximately 1.0 to 1.02 grams per cubic centimeter at twenty degrees Celsius (Sigma-Aldrich, 2024; TCI Chemicals, 2024)
What is Isobutyl Phenylacetate?
Isobutyl Phenylacetate, systematically designated as 2-methylpropyl phenylacetate, represents a member of the phenylacetic acid ester family within aromatic aliphatic compounds. This synthetic ester belongs to the broader class of benzeneacetic acid derivatives and functions as both a fragrance material and flavoring substance. The compound consists of a phenylacetic acid moiety esterified with isobutanol, yielding a molecule that bridges aromatic and aliphatic structural characteristics.
Chemically classified as an aryl-aliphatic ester, Isobutyl Phenylacetate exhibits intermediate volatility positioning it primarily within the heart note category of perfume construction, though its sweet honey-cocoa character can extend into both top and base note territories depending on formulation context. The presence of the branched isobutyl group contributes to its distinctive olfactory profile while providing moderate hydrophobicity and good blending characteristics with both polar and non-polar fragrance components.
As a synthetic aromatic ester, this material finds extensive application across both fine fragrance and functional perfumery sectors, particularly valued for its ability to contribute authentic honey-cocoa tonalities without the instability associated with certain natural extracts. The compound demonstrates good stability under typical storage and formulation conditions, though like most esters, it may undergo gradual hydrolysis in highly acidic or alkaline environments over extended periods.
Historical Background
The development of phenylacetic acid esters as perfumery materials emerged during the late nineteenth and early twentieth centuries, coinciding with the broader expansion of synthetic organic chemistry and its application to fragrance creation. Phenylacetic acid itself was first isolated and characterized in the mid-1800s, with its esterification products following as chemists explored structure-odor relationships within aromatic compounds.
Isobutyl Phenylacetate specifically represents part of the systematic exploration of alkyl phenylacetates that occurred during the early decades of the twentieth century. Industrial chemists at major fragrance houses, particularly in France, Germany, and Switzerland, systematically prepared various alkyl esters of phenylacetic acid to map their olfactory properties. This compound emerged as particularly valuable due to its distinctive honey-cocoa character, which differentiated it from its homologs such as methyl phenylacetate (more intensely honey-floral) and phenylethyl phenylacetate (more rosy-fruity).
The synthesis methodology follows classical esterification chemistry developed by Emil Fischer and Arthur Speier in the 1890s (Fischer & Speier, 1895). The Fischer-Speier esterification process, involving direct condensation of carboxylic acids with alcohols under acidic catalysis, provided the foundational synthetic route for producing this compound industrially. This method remains the primary production pathway today, with refinements in catalyst systems and purification techniques enhancing yield and purity.
By the mid-twentieth century, Isobutyl Phenylacetate had become established within both flavor and fragrance industries. Its dual-use nature and distinctive organoleptic properties secured its position in formulations ranging from fine perfumes to confectionery flavorings. The compound received official recognition through its assignment of FEMA number 2210, indicating approval for use in food flavoring applications under specified conditions (FEMA, 2024).
Throughout the latter half of the twentieth century and into the twenty-first century, this material has maintained steady utilization, particularly in applications requiring authentic cocoa-honey effects. Its classification under multiple regulatory frameworks, including European Union food and cosmetic regulations, demonstrates its continued commercial relevance and safety profile established through decades of use.
Olfactory Profile
Scent Family: Sweet floral-gourmand with pronounced honey-cocoa facets; classified within the balsamic-sweet olfactory group with secondary floral characteristics.
Main Descriptors: The olfactory profile of Isobutyl Phenylacetate centers on a rich, sweet honey-cocoa accord that immediately distinguishes it from other phenylacetate esters. The primary impression combines dark cocoa powder sweetness with warm honey tonalities, creating a confectionery-like effect that reads as both comforting and indulgent. Underlying this dominant theme are subtle floral nuances that prevent the profile from becoming purely gourmand, instead maintaining a sophisticated balance between edible sweetness and perfumistic elegance.
At full strength, the material opens with immediate sweetness reminiscent of honeycomb and milk chocolate, accompanied by waxy-balsamic undertones. As the scent develops on a smelling strip, fruity facets emerge, suggesting ripe pear and stone fruits macerated in honey. A subtle spicy nuance, likely derived from the phenylacetic acid backbone, adds complexity and prevents monotony. The overall effect is rounded, soft, and enveloping, lacking the sharp or harsh edges sometimes present in purely synthetic materials.
When evaluated at one percent dilution in perfumer's alcohol, Isobutyl Phenylacetate reveals additional subtleties, including a mild musk-like undertone that enhances its blending capabilities. The cocoa aspect becomes more refined at lower concentrations, reading less as chocolate per se and more as a sophisticated cacao-blossom note. At ten percent dilution, the full honey-cocoa character dominates, suitable for creating impactful gourmand accords or enriching oriental and amber bases.
Intensity: Medium-strong; the material possesses sufficient diffusion to impact fragrance compositions at relatively low inclusion levels, typically effective between 0.5 and five percent in fine fragrance formulations. In flavor applications, it demonstrates high impact even at concentrations below ten parts per million, with taste characteristics described as sweet, cocoa, fruity, honey, and waxy with spicy nuances becoming perceptible at four parts per million (ChemicalBook, 2024).
Tenacity: Moderate to good; Isobutyl Phenylacetate exhibits persistence on smelling strips for approximately twelve to twenty-four hours, positioning it primarily as a heart note material with some base note character. The ester linkage provides sufficient volatility to contribute to early development phases while the molecular weight (192.25 grams per mole) and lipophilic character ensure reasonable longevity. In alcohol-based formulations, the material demonstrates good substantivity, though like most esters, it benefits from fixation with heavier materials such as musks, amber bases, or benzoin derivatives.
Volatility: Medium; the compound occupies the heart note category within traditional perfumery classification, with evaporation rates slower than typical top note materials (such as citruses or light aldehydes) but faster than base notes (such as woody materials or animalic musks). This positioning makes it valuable for building the middle development phase of fragrances, where it can bridge lighter opening notes with heavier drydown materials. The branched isobutyl group reduces volatility compared to straight-chain butyl homologs while maintaining sufficient lift to prevent excessive heaviness.
Applications in Fine Fragrance
Within fine fragrance composition, Isobutyl Phenylacetate serves primarily as a honey-cocoa character builder and gourmand modifier. Its distinctive sweet profile makes it particularly valuable in contemporary perfumery's embrace of edible and comfort-driven olfactory themes. The material excels in oriental fragrances, where its balsamic-sweet character harmonizes with amber, vanilla, and spice elements to create rich, enveloping effects. In floral-oriental compositions, it can soften and sweeten heavier florals such as jasmine, tuberose, or ylang-ylang, adding gourmand warmth without overwhelming the floral heart.
The compound demonstrates excellent synergy with other sweet materials, including vanillin, ethyl maltol, and various lactones, allowing perfumers to build complex gourmand structures. When combined with Methyl Phenylacetate, it creates sophisticated honey accords that possess both the sharp, heady intensity of methyl phenylacetate and the rounded, cocoa-like depth of the isobutyl ester. This pairing often appears in honey reconstructions and in fragrances seeking to evoke beeswax, honeycomb, or sweetened florals.
In modern fragrance design, Isobutyl Phenylacetate finds application in gourmand compositions featuring chocolate, caramel, or praline themes. It pairs effectively with cocoa-related materials such as pyrazines, furan derivatives, and chocolate absolute, contributing authentic cocoa butter sweetness. The material also supports fruity-floral constructions, particularly those featuring stone fruits (peach, apricot, plum), where its waxy-fruity facets complement fruit esters while adding substantive depth.
The compound works well within chypre structures, particularly modern interpretations that incorporate gourmand elements. Here, it can bridge the contrast between fresh citrus-aromatic top notes and woody-mossy bases, adding warmth and accessibility. Similarly, in fougère compositions, it contributes unexpected sweetness that modernizes traditional frameworks, creating hybridized fougère-gourmand structures increasingly popular in contemporary masculine and unisex fragrances.
Performance in Formula
In practical formulation work, Isobutyl Phenylacetate demonstrates good stability and compatibility with most fragrance ingredients. The material blends readily in alcohol-based formulations (typical ethanol concentrations of seventy to ninety-five percent) and shows good solubility in most perfumery solvents. It exhibits moderate stability in soap bases, though like most esters, some loss can occur during saponification or in highly alkaline environments. In detergent applications, the compound's performance depends on pH and temperature conditions, with best results achieved in pH-neutral to slightly acidic formulations.
The ester demonstrates photostability adequate for most applications, though prolonged exposure to direct sunlight may cause gradual discoloration in concentrated form. In finished fragrances containing appropriate antioxidants and ultraviolet stabilizers, this presents minimal concern. The material maintains its olfactory character across a wide range of temperatures, making it suitable for both cool and warm climate formulations, though excessive heat during storage should be avoided to prevent accelerated hydrolysis.
When formulating with Isobutyl Phenylacetate, perfumers should note its tendency to amplify sweet notes in composition. At concentrations above three to five percent in fine fragrance, the honey-cocoa character can become dominant, potentially overshadowing more delicate floral or citrus components. Strategic use at lower levels (0.5 to two percent) allows the material to enhance sweetness and add depth without overwhelming compositional balance. The compound responds well to fixation with materials such as Benzyl Acetate, heliotropine, or synthetic musks, which can extend its longevity and integrate its character more seamlessly into complex accords.
Industrial & Technical Uses
Beyond fine fragrance applications, Isobutyl Phenylacetate serves multiple technical functions across flavor and fragrance industries. In flavor creation, it contributes honey-chocolate character to confectionery formulations, appearing in chocolate flavors, caramel profiles, and honey reconstructions. The compound finds use in dairy flavor systems, where it can enhance creamy, buttery notes while adding subtle chocolate undertones. Its approval under FEMA GRAS status (FEMA 2210) permits its use in food applications at appropriate levels, typically below ten parts per million in finished products (FEMA, 2024).
In functional perfumery, the material appears in household products including fabric softeners, air fresheners, and personal care items where honey-vanilla characters are desired. Its moderate cost relative to some natural honey extracts makes it economically attractive for mass-market applications. The compound's stability profile suits incorporation into various consumer product matrices, including aqueous and emulsion systems common in personal care formulations.
From a technical perspective, Isobutyl Phenylacetate serves as a reference standard in analytical chemistry, particularly for gas chromatography and mass spectrometry applications. Its well-defined structure and characteristic fragmentation pattern make it useful for method development and instrument calibration in flavor and fragrance analysis laboratories.
Regulatory & Safety Overview
IFRA Status: According to the International Fragrance Association Standards, Isobutyl Phenylacetate is not currently subject to specific restrictions under IFRA Amendment 51 (IFRA, 2023). The material may be used in fragrance compositions without quantitative limitations, though formulators should always consult the most current IFRA Standards Library for any updates. Products should comply with general IFRA Code of Practice principles regarding good manufacturing practices and appropriate quality control. For the latest regulatory information, visit the IFRA Standards Library.
EU Cosmetics Regulation: Under EU Regulation (EC) No 1223/2009, Isobutyl Phenylacetate is permitted for use in cosmetic and personal care products (European Commission, 2009). The compound does not appear among the twenty-six mandatory declarable allergens listed in Annex III of the regulation, eliminating specific labeling requirements related to allergenicity. However, as with all fragrance materials, it must be used in accordance with safety assessments and good manufacturing practices. The material's EC number (203-007-9) facilitates its identification within European regulatory frameworks.
EU Food Regulation: The compound holds approval for food flavoring use under EU Regulation (EC) No 1334/2008 and its amendments, including Regulation (EU) No 872/2012 (European Commission, 2008, 2012). Its Flavis number (09.788) provides traceability within European flavor ingredient databases. Use in food applications must comply with maximum usage levels and good manufacturing practices as specified in relevant European legislation.
FEMA Status: The Flavor and Extract Manufacturers Association recognizes Isobutyl Phenylacetate under FEMA number 2210 as Generally Recognized as Safe (GRAS) for use in food flavoring applications (FEMA, 2024). This status, based on expert review of safety data and history of use, permits the compound's inclusion in flavor formulations at levels consistent with good manufacturing practices and within established use levels. Typical application concentrations in finished food products range from one to ten parts per million depending on the specific flavor system and target application.
FDA Status: In the United States, the material is regulated under FDA 21 CFR 172.515 (synthetic flavoring substances) and FDA 21 CFR 117 (current good manufacturing practice regulations). These regulations govern its use in food contact applications and set forth requirements for manufacturing, processing, and labeling.
Other Regulatory Considerations: The compound maintains Halal and Kosher certification from relevant certifying bodies when produced under appropriate conditions, making it suitable for formulations requiring such compliance (Sigma-Aldrich, 2024). It meets purity specifications established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), demonstrating conformance to international food safety standards.
Toxicology: Safety assessment data indicates low toxicity potential for Isobutyl Phenylacetate when used at appropriate levels in fragrance and flavor applications. The compound has been evaluated for skin sensitization potential, with results supporting its safe use in topical applications at concentrations typical in perfumery and cosmetics. As with all fragrance materials, appropriate handling procedures should be followed during manufacturing and formulation, including use of adequate ventilation and personal protective equipment when handling concentrated material. The material should be stored in tightly closed containers away from heat sources and incompatible materials such as strong oxidizers.
References
ChemicalBook. (2024). Phenylacetic acid isobutyl ester (102-13-6). Retrieved from https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2752314.htm
European Commission. (2008). Regulation (EC) No 1334/2008 on flavourings and certain food ingredients with flavouring properties. Official Journal of the European Union, L 354/34.
European Commission. (2009). Regulation (EC) No 1223/2009 on cosmetic products. Official Journal of the European Union, L 342/59.
European Commission. (2012). Regulation (EU) No 872/2012 amending Regulation (EC) No 1334/2008. Official Journal of the European Union, L 267/1.
Flavor and Extract Manufacturers Association (FEMA). (2024). FEMA GRAS assessment program. Retrieved from https://www.femaflavor.org
Fischer, E., & Speier, A. (1895). Darstellung der Ester. Chemische Berichte, 28, 3252-3258.
International Fragrance Association (IFRA). (2023). IFRA Standards – 51st Amendment. Geneva: International Fragrance Association. Retrieved from https://ifrafragrance.org/safe-use/library
PubChem. (2024). Isobutyl phenylacetate (CID 60998). National Center for Biotechnology Information. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Isobutyl-phenylacetate
Sigma-Aldrich. (2024). Isobutyl phenylacetate ≥98%, FCC, FG (CAS 102-13-6). Retrieved from https://www.sigmaaldrich.com/US/en/product/aldrich/w221007
Surburg, H., & Panten, J. (2016). Common fragrance and flavor materials: Preparation, properties and uses (6th ed.). Wiley-VCH Verlag.
TCI Chemicals. (2024). Isobutyl phenylacetate (Product No: P0124). Retrieved from https://www.tcichemicals.com/US/en/p/P0124