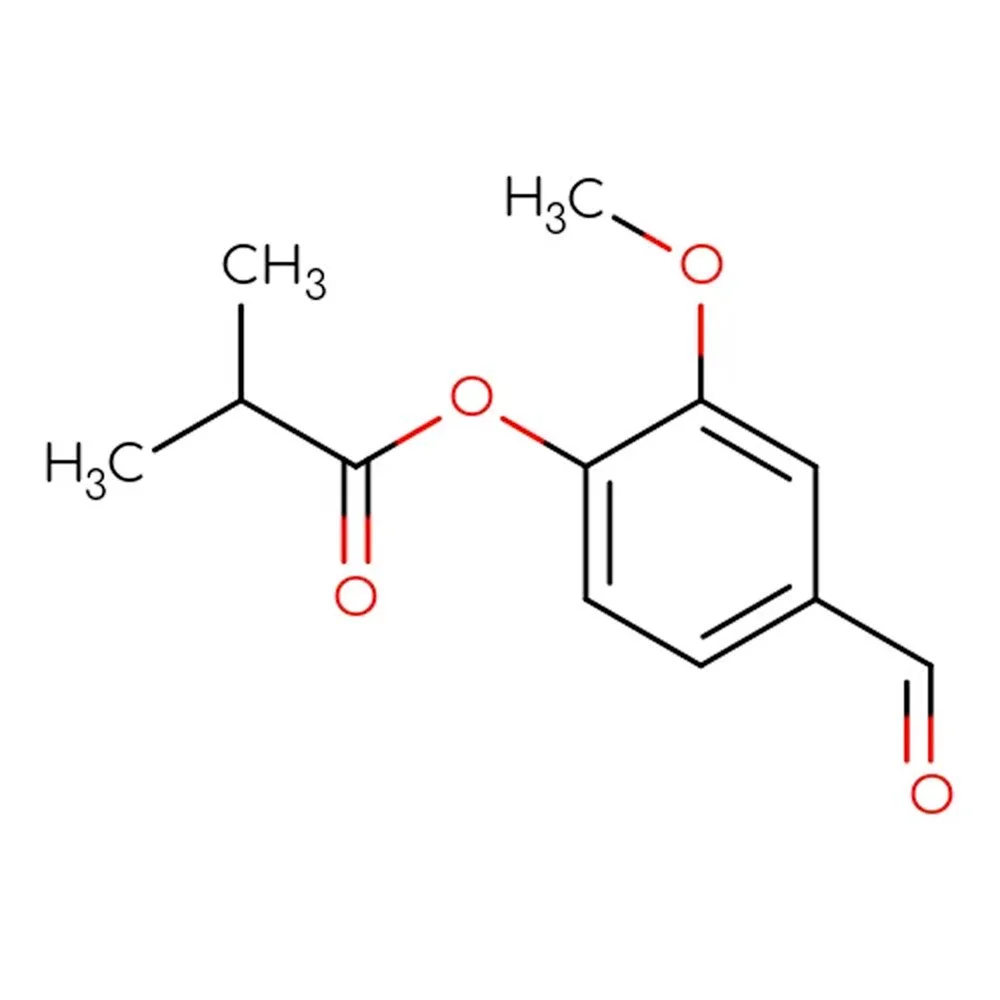
ISOBUTAVAN - Technical Ingredient Overview
🔎 Chemical Name — 4-Formyl-2-methoxyphenyl 2-methylpropanoate; Vanillin isobutyrate
🧪 Synonyms — Vanillyl isobutyrate; Vanilliyl isobutanoate; Isobutyl lignate; 3-Methoxy-4-isobutyryloxybenzaldehyde
📂 CAS Number — 20665-85-4
📘 FEMA Number — 3754 (BC Fragrance, 2025)
⚖️ Molecular Weight — 222.24 g/mol
📝 Odor Type — Vanillic, Gourmand
📈 Odor Strength — Moderate to Strong
👃🏼 Odor Profile — Sweet, creamy vanillic character with distinctive white chocolate facets, cream soda nuances, and soft apricot fruitiness. Less powdery than vanillin with a thicker, more buttery texture
⚗️ Uses — Color-stable vanillin substitute for white soaps and light-colored formulations; gourmand modifier for fragrances; fixative in fine perfumery
🧴 Appearance — Colorless to pale yellow liquid
What is Isobutavan?
Isobutavan is a synthetic vanillic compound created by esterifying vanillin with isobutyric acid. This modification "protects" the reactive phenolic hydroxyl group of vanillin, dramatically improving chemical stability while preserving the beloved vanilla character. The resulting molecule belongs to the vanillic-balsamic olfactory family and serves as a high-performance alternative to vanillin in applications where color stability and longevity are critical.
Unlike natural vanillin (which reacts with trace metals and alkaline conditions to produce brown or purple discoloration), Isobutavan remains color-stable in challenging formulations including white soaps, alkaline shampoos, and pale lotions. This stability stems from the isobutyrate ester group, which prevents the oxidation and metal complexation reactions that cause vanillin's notorious discoloration issues (Pybus & Sell, 2006).
The compound offers perfumers a creamy, rounded vanilla note with gourmand richness that differs from vanillin's sharper, more powdery profile. Its enhanced substantivity (lasting over 400 hours on a blotter) makes it valuable as both a modifier and fixative in modern perfumery.
Historical Background
Isobutavan was developed by Quest International (now part of Givaudan) as part of a broader effort to solve perfumery's "vanillin problem"—the tendency of vanillin to discolor products, particularly soaps and emulsions. This challenge became acute with the rise of "trickle-down" marketing in the 1980s, where fine fragrances were adapted into full product lines including soaps, lotions, and functional products. Many prestigious fragrances contained substantial vanillin, making reformulation for white soaps nearly impossible without losing the signature character (Pybus & Sell, 2006).
The solution came through ester chemistry. By protecting vanillin's phenolic group with an isobutyrate ester, Quest's chemists created a molecule that retained vanillic sweetness while eliminating reactivity. The isobutyrate group was specifically chosen for its balance of stability, olfactory contribution, and synthetic accessibility—it adds subtle creamy-fruity nuances that complement rather than mask the vanilla character.
Isobutavan was commercialized in the late 1980s to early 1990s and quickly became indispensable for soap perfumers. Its development paralleled similar "protected vanillin" derivatives including Ultravanil® (where the aldehyde is reduced to a methyl group), demonstrating the industry's systematic approach to solving formulation challenges through molecular modification.
The compound's trade name "Isobutavan" cleverly combines "isobutyrate" with "vanillin," immediately communicating both its chemical structure and olfactory character to perfumers.
Olfactory Profile
Scent Family: Vanillic, Gourmand, Balsamic
Main Descriptors: Isobutavan presents a sweet, creamy vanilla aroma with pronounced gourmand characteristics. The scent profile centers on white chocolate and cream soda notes, softer and rounder than vanillin's classic vanilla-powder character. A distinctive apricot-fruity nuance adds brightness, while subtle nutmeg-spicy undertones provide complexity. The overall impression is rich, buttery, and indulgent—less sharp and medicinal than vanillin, with a fuller, more "mouthfeel"-like texture that perfumers describe as "thickening" a composition (Givaudan, 2025).
Unlike vanillin's relatively linear progression, Isobutavan develops through multiple phases. The initial impression emphasizes creamy sweetness with prominent white chocolate facets. As it develops, caramel and butterscotch notes emerge, supported by a subtle balsamic-woody base that adds sophistication. The apricot-fruity aspect provides lift without turning overtly fruity, maintaining the composition's gourmand integrity.
Intensity: Moderately powerful with good diffusion. Requires less dosage than vanillin to achieve comparable sweetness—typical usage ranges from 0.15-2% in fragrance concentrates. The ester structure moderates volatility compared to the free aldehyde, resulting in a more controlled, sustained release of vanilla character (BC Fragrance, 2025).
Tenacity: Exceptional longevity, lasting over 400 hours on a smelling strip—significantly superior to vanillin's 48-72 hour persistence. On skin, Isobutavan maintains presence for 8-12 hours, functioning effectively as a fixative while continuing to contribute olfactory character throughout wear. The extended tenacity derives from the molecule's higher molecular weight and lipophilicity compared to vanillin.
Volatility: Functions primarily as a base note to heart note ingredient. The protected aldehyde structure reduces top-note volatility while the isobutyrate group increases molecular weight (MW 222 vs vanillin's 152), shifting the evaporation profile downward. This makes Isobutavan particularly effective in the mid-to-late development stages where vanilla sweetness traditionally fades.
Applications in Fine Fragrance
Isobutavan excels in gourmand fragrances where creamy vanilla depth is desired without the powderiness of straight vanillin. The compound's white chocolate character makes it indispensable for confectionery-inspired accords, pairing naturally with praline notes, caramel effects, and dairy nuances. In modern oriental compositions, it provides sweet warmth that complements amber, benzoin, and tonka bean.
The material works effectively in floral fragrances to add roundness and diffusion without dominating—a few drops can transform a composition by providing creamy body that supports rather than overshadows floral notes. The soft apricot facet creates interesting bridges to fruity accords, particularly stone fruits and tropical notes.
Isobutavan blends exceptionally well with other vanillic materials (vanillin, ethyl vanillin, heliotropin), creating more complex and natural-seeming vanilla effects through layering. It pairs beautifully with lactones (gamma-decalactone, gamma-undecalactone) for dairy-cream effects, coumarin for sweet-hay nuances, and woody materials (sandalwood, cedarwood) for sophisticated gourmand-woody accords.
Performance in Formula
The key advantage of Isobutavan lies in its color stability. While vanillin reacts with iron traces to produce purple discoloration and degrades in alkaline conditions to form brown compounds, Isobutavan can be used up to 2% in white soap formulations before any color shift becomes noticeable (Givaudan, 2025). This makes it the go-to choice for trickle-down formulations where maintaining color is essential.
The compound demonstrates good stability across most cosmetic bases including alcoholic perfumes, emulsions, shampoos, and conditioners. However, it shows reduced stability in strongly alkaline systems (pH >10) such as household cleaners and bleaches, where gradual ester hydrolysis can occur, regenerating vanillin and isobutyric acid. Performance in acidic formulations is excellent.
Solubility characteristics favor lipophilic systems—Isobutavan dissolves readily in alcohol, oils, and most perfumery solvents, with limited water solubility. The compound integrates smoothly into both fine fragrance concentrates and functional fragrance applications.
Industrial & Technical Uses
Beyond perfumery, Isobutavan finds application in flavor formulations where FEMA 3754 status permits its use in food products. Typical applications include vanilla-flavored confectionery, baked goods, beverages, and dairy products, though usage levels remain modest (typically <2 ppm) due to cost and the availability of more traditional vanillin for flavor work.
In personal care, the compound appears in premium formulations requiring both vanilla character and visual appeal—white creams, light-colored lotions, and translucent gels benefit from its non-discoloring properties. The material's fixative qualities support other volatile ingredients, extending overall fragrance performance in leave-on products.
Regulatory & Safety Overview
IFRA Status: Isobutavan is unrestricted under IFRA Amendment 51 across all product categories. This clean regulatory profile represents a significant advantage over vanillin, which faces concentration limits in certain applications. The absence of restrictions reflects favorable toxicological data and the molecule's chemical stability, which prevents formation of sensitizing oxidation products.
EU Cosmetics Regulation: Compliant for cosmetic use without specific restrictions under EU Regulation 1223/2009. Not classified as a skin sensitizer or allergen requiring declaration. The compound's protected structure eliminates the reactivity concerns associated with free phenolic aldehydes.
FEMA Status: FEMA 3754, affirmed as acceptable for use as a flavoring substance. While GRAS (Generally Recognized as Safe) status details remain unpublished, the FEMA listing indicates expert panel review and acceptance for intended flavor applications under standard usage conditions.
Safety Profile: Low toxicity with favorable dermal and oral safety profiles. The compound is non-volatile enough to present minimal inhalation concerns. Standard handling precautions for perfumery materials apply—avoid direct contact with eyes and prolonged skin exposure to concentrated material. Unlike vanillin, Isobutavan does not present significant allergy concerns in finished products at typical use levels.
GHS Classification: Not classified as hazardous under globally harmonized system criteria. Non-flammable under normal conditions (flash point 101°C), though combustible at elevated temperatures. No special transport restrictions apply.
References
ChemBK. (2025). Vanillin isobutyrate. Retrieved October 26, 2025, from https://www.chembk.com/en/chem/Vanillin Isobutyrate
ChemicalBook. (2025). Vanillin isobutyrate (20665-85-4). Retrieved October 26, 2025, from https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0127044.htm
Givaudan. (2025). Isobutavan [Product description]. Retrieved October 26, 2025, from https://www.givaudan.com/fragrance-beauty/fragrance-ingredients-business/fragrance-molecules/isobutavan
PubChem. (2025). PubChem Compound Summary for CID 539829, Vanillin isobutyrate. Retrieved October 26, 2025, from https://pubchem.ncbi.nlm.nih.gov/compound/Vanillin-isobutyrate
Pybus, D. H., & Sell, C. S. (Eds.). (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Royal Society of Chemistry.