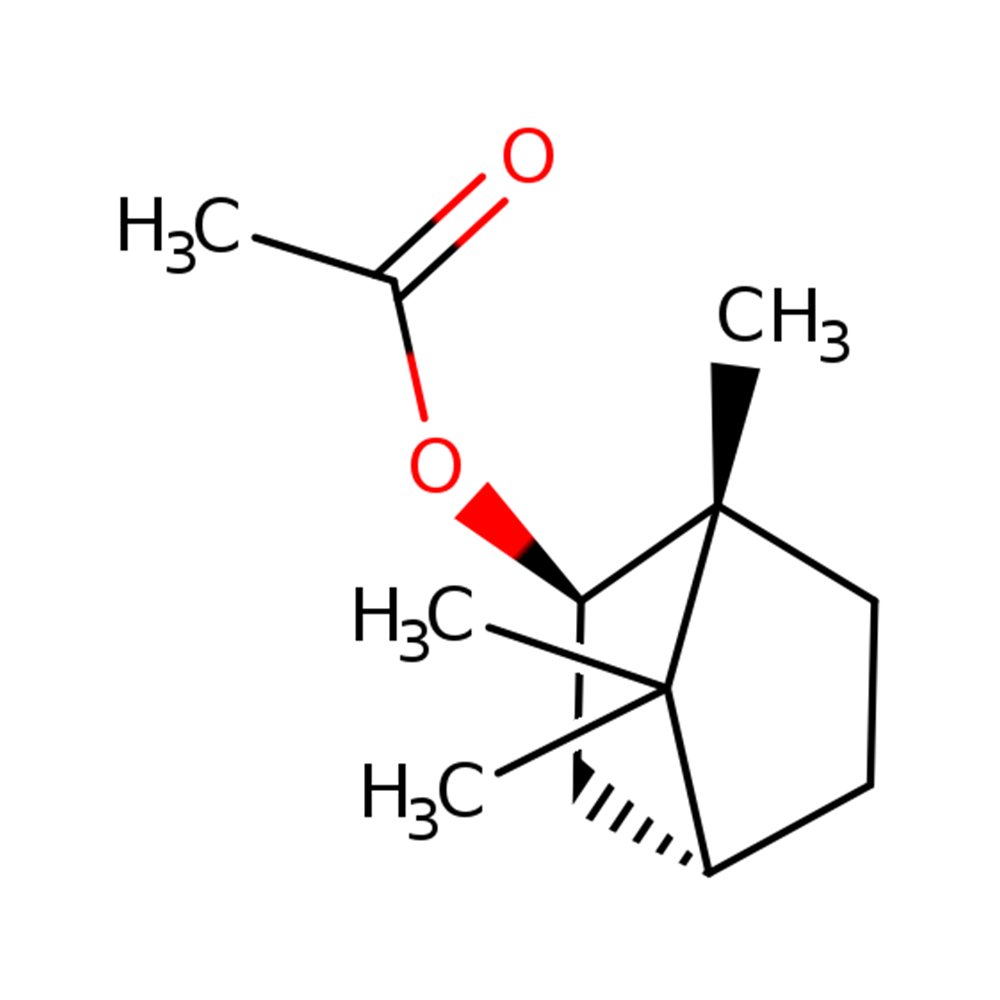
ISOBORNYL ACETATE (125-12-2) Technical Ingredient Overview
🔎 Chemical Name — Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, exo-
🧪 Synonyms — Isobornyl acetate, 2-exo-bornanyl acetate, Acetic acid isobornyl ester, exo-1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate, Pichtosin, Pichtosine, 2-Camphanyl acetate, exo-Bornyl acetate
📂 CAS Number — 125-12-2
📘 FEMA Number — 2160
⚖️ Molecular Weight — 196.29 g/mol
📝 Odor Type — Fresh, Terpenic, Woody-Camphoraceous
📈 Odor Strength — Medium (approximately 8 hours on strip when undiluted)
👃🏼 Odor Profile — Fresh, woody, dry-amber character with green pine and camphoraceous nuances; reminiscent of borneol with a pleasant pine-needle odor; slightly medicinal and minty undertone with characteristic hemlock-like aspects (Arctander, 1994; Scentspiracy, 2024)
⚗️ Uses — Fragrance modifier, herbalizer, fougère base construction, soap and detergent perfumery, household products, camphor synthesis intermediate, FEMA GRAS-approved flavoring agent
🧴 Appearance — Colorless to pale straw yellow liquid (Arctander, 1994; Chemicalbook, 2024)
What is Isobornyl Acetate?
Isobornyl Acetate is a synthetic terpenic ester derived from the acetylation of isoborneol or through catalytic addition of acetic acid to camphene. It belongs to the bicyclic monoterpene family and represents the exo-isomer of bornyl acetate, distinguished by its specific stereochemistry at the bridgehead positions of the bicyclo[2.2.1]heptane (norbornane) skeleton (Pybus & Sell, 2006).
While trace amounts of isobornyl acetate have been identified in various essential oils including fir needle, basil (Ocimum basilicum), rosemary, thyme, dill herb, and even in food products like Parmesan cheese and custard apple, commercial isobornyl acetate is produced synthetically to ensure consistent olfactory quality, high purity, and cost-effectiveness (Arctander, 1994; Chemicalbook, 2024). The synthetic material is typically optically inactive, though it may exhibit slight optical activity depending on the stereochemistry of the starting terpene material.
Chemically, isobornyl acetate features a bicyclic structure with a seven-membered norbornane framework bearing three methyl substituents and an acetate ester functionality at the C-2 position in the exo-configuration. This specific stereochemistry differentiates it from its endo-isomer, bornyl acetate, and contributes to its distinct olfactory profile and physical properties.
Historical Background
The development of isobornyl acetate is intimately connected to the broader history of terpene chemistry and the industrial utilization of pinene-derived materials. The compound emerged from systematic investigations into the Wagner-Meerwein rearrangement—a fundamental carbocationic rearrangement discovered in the late 19th century through studies of pinene and camphene chemistry (Pybus & Sell, 2006).
The synthetic route from camphene to isobornyl acetate was elucidated as researchers explored acid-catalyzed additions to terpene hydrocarbons. Early synthesis methods employed sulfuric acid as a catalyst for the addition of acetic acid to camphene, a reaction that proceeds through carbocationic intermediates characteristic of the Wagner-Meerwein rearrangement mechanism (Arctander, 1994; Pybus & Sell, 2006). This discovery allowed for the large-scale production of isobornyl acetate from abundant pinene-derived feedstocks, particularly turpentine fractions rich in α-pinene that could be isomerized to camphene.
The industrial significance of isobornyl acetate grew substantially in the early-to-mid 20th century, driven by two primary applications: as a fragrance material for household products and soaps, and critically, as an intermediate for camphor synthesis. The latter application became particularly important when natural camphor supplies from camphor laurel (Cinnamomum camphora) became insufficient to meet industrial demand. Hydrolysis of isobornyl acetate yields isoborneol, which can be oxidized to produce synthetic camphor—a compound essential for plastics manufacturing, pharmaceuticals, and other industrial applications (Pybus & Sell, 2006).
By the mid-20th century, refinements in catalytic systems improved both yield and selectivity. The introduction of styrene-divinylbenzene acid ion-exchange resins as catalysts offered advantages over traditional mineral acid catalysts, including easier product recovery and reduced equipment corrosion (Arctander, 1994; Ning et al., 2012). These technological advances consolidated isobornyl acetate's position as a major commodity fragrance chemical.
Olfactory Profile
Scent Family
Woody-Terpenic with Camphoraceous-Green subcategory
Main Descriptors
Primary: Fresh, dry-woody, pine-needle, camphoraceous
Secondary: Green-herbal, slightly amber, balsamic undertones
Tertiary: Medicinal-minty nuances, hemlock-like aspects, clean and crisp
The olfactory character of isobornyl acetate is defined by its crisp, forest-like freshness that evokes coniferous needles and resinous wood. Unlike α-terpineol or terpinen-4-ol, which skew citrusy or phenolic, isobornyl acetate maintains a distinctly dry, non-fruity character that provides structural support rather than decorative flourish (Scentspiracy, 2024). Its camphoraceous facet is less pronounced than in camphor itself but more evident than in other pine-derived materials, creating a balanced woody-green impression.
Intensity
Medium intensity with moderate diffusivity. On a perfumer's blotter, undiluted isobornyl acetate typically persists for approximately 8 hours, demonstrating good substantivity without overwhelming dominance (Scentspiracy, 2024).
Tenacity
Moderate longevity. The compound exhibits good tenacity for a moderately volatile terpenic ester, bridging the gap between fleeting top notes and persistent base materials. Its acetate ester functionality provides sufficient volatility for initial impact while the bicyclic structure imparts relative stability and extended presence.
Volatility
Classified as a middle-to-top note material with moderate volatility. The boiling point of 102-103°C at 1.6-1.7 kPa (Arctander, 1994) positions it in the intermediate volatility range—more volatile than heavy woods or balsams but less fleeting than monoterpene hydrocarbons or light aldehydes. This balanced volatility profile makes it particularly useful for bridging olfactory registers in complex compositions.
Fixative Role
While not a traditional fixative in the sense of heavy musks or resins, isobornyl acetate contributes to fragrance structure through its moderate tenacity and blending power. In soap and detergent applications, it provides diffusive freshness that persists through the product lifecycle. In fine fragrance, it functions as a structural modifier that enhances the clarity and definition of green-woody accords without adding excessive weight.
Applications in Fine Fragrance
Isobornyl acetate serves as a versatile building block in modern perfumery, valued for its ability to create dimensional freshness and structural support in green, herbal, and woody compositions.
Primary Roles
Fougère Accords: Essential for constructing classic fougère structures, where its dry-herbal character bridges aromatic lavender notes with woody-coumarinic bases
Coniferous/Forest Notes: Fundamental in pine-needle, fir balsam, and forest-floor reconstructions where its authentic evergreen character provides realism
Green Modifiers: Used to add crispness and natural "outdoor" freshness to floral bouquets, preventing them from becoming overly sweet or heavy
Herbal-Aromatic Complexes: Contributes to rosemary, thyme, and mixed-herb effects through its characteristic terpenic-camphoraceous profile
Typical Pairings
With Lavender & Lavandin: Creates herbaceous-aromatic complexity; enhances the camphoraceous facets of lavender oils
With Coumarin & Tonka: Provides freshness that prevents fougère bases from becoming too sweet or vanillic
With Cedarwood & Vetiver: Adds green, living-wood aspects to dry woody bases
With Citrus: Extends and naturalizes citrus top notes while adding depth
With Pine & Fir Oils: Reinforces and clarifies natural conifer materials
Behavior in Blends
Isobornyl acetate demonstrates excellent blending behavior, integrating smoothly without dominating or clashing. Its relatively neutral character allows it to support other materials rather than competing for attention. In skilled formulation, it lends structural clarity and "breathing room" to otherwise dense or heavy compositions, creating what perfumers describe as dimensional freshness—the perception of airiness and natural vitality.
Performance in Formula
Isobornyl acetate exhibits favorable technical properties that facilitate its use across diverse product applications:
Stability: Good stability in both alcoholic and soap bases; moderate photostability though protection from prolonged UV exposure is recommended
Solubility: Readily soluble in ethanol and other common perfumery solvents; insoluble in water (approximately 9.7-160 mg/L water solubility reported) (ECHA, n.d.)
pH Tolerance: Stable across typical cosmetic pH ranges; acetate ester linkage is susceptible to hydrolysis under strongly alkaline conditions
Compatibility: Excellent compatibility with most fragrance materials; does not cause discoloration or precipitation in standard formulations
Diffusion: Moderate diffusivity that provides balanced evaporation across application types
The compound's moderate volatility makes it particularly well-suited for products requiring sustained freshness—soaps, detergents, air fresheners, and fabric care items benefit from its ability to provide immediate impact with extended presence.
Industrial & Technical Uses
Beyond fine fragrance, isobornyl acetate serves several important industrial functions:
Camphor Synthesis
The major industrial use of isobornyl acetate remains as an intermediate for synthetic camphor production. Hydrolysis yields isoborneol, which undergoes oxidation to camphor—a compound essential for:
Plastics manufacturing (cellulose nitrate and cellulose acetate)
Pharmaceutical applications
Moth repellents and preservatives
Industrial solvents
Household & Personal Care
Extensively used in:
Laundry detergents and fabric softeners
Hard-surface cleaners and bathroom products
Air fresheners and odor-control systems
Bath preparations and soap bars
Shampoos and shower gels
Flavor Applications
As a FEMA GRAS (Generally Recognized as Safe) approved flavoring agent (FEMA #2160), isobornyl acetate finds limited use in food flavoring at concentrations typically ranging from 2-10 ppm, where it contributes camphoraceous, woody, and slightly citrus notes (FEMA, n.d.; Chemicalbook, 2024).
Antimicrobial Applications
Recent research has demonstrated that isobornyl acetate possesses antimicrobial properties, showing activity against various bacteria (including methicillin-resistant Staphylococcus aureus), fungi (Candida albicans), and some viruses. These properties make it of interest in preservative-free and hygiene-forward product development, though its primary commercial use remains fragrance-driven (Chemicalbook, 2024).
Insect Behavior Modification
Like many plant-derived terpenes, isobornyl acetate exhibits antifeedant properties that deter certain insect species, contributing to its historical use in natural product-based pest management strategies (Wikipedia, 2025).
Regulatory & Safety Overview
IFRA Status
Isobornyl acetate is not specifically restricted under IFRA Standards up to and including Amendment 51 (notified June 30, 2023). It does not appear in the list of prohibited or restricted materials, indicating it may be used without category-specific concentration limits beyond good manufacturing practices (IFRA, 2023). Formulators should always verify the latest IFRA Standards for updates.
Official IFRA Documentation: IFRA Standards Library
EU Cosmetics Regulation
Under EU Regulation (EC) No 1223/2009, isobornyl acetate is permitted for use in cosmetic products. It does not appear in Annex II (prohibited substances), Annex III (restricted substances), or other restrictive annexes of the Cosmetics Regulation. The ingredient is listed in the INCI (International Nomenclature of Cosmetic Ingredients) system as "ISOBORNYL ACETATE" and is registered in the CosIng database, confirming its acceptability for EU cosmetic formulations (COSMILE Europe, 2023).
EU Cosmetics Regulation: Regulation (EC) No 1223/2009
EC Number: 204-727-6
FEMA GRAS Status
Isobornyl acetate holds FEMA GRAS (Generally Recognized as Safe) status for use as a flavoring agent under FEMA Number 2160. The material has been evaluated by the Flavor and Extract Manufacturers Association Expert Panel and is approved for food and beverage applications at appropriate use levels (FEMA, n.d.; Sigma-Aldrich, n.d.).
Council of Europe Number: 2066
Flavis Number: 9.218
REACH Registration
Isobornyl acetate is registered under the EU REACH Regulation (EC) No 1907/2006, indicating that manufacturers and importers have submitted the required safety and toxicological data to the European Chemicals Agency (ECHA). Registration confirms compliance with European chemical safety requirements for production and marketing volumes exceeding 1 tonne per year (ECHA, n.d.).
Toxicology & Safety
The toxicological profile of isobornyl acetate indicates low hazard under normal use conditions:
Acute Toxicity: Low oral and dermal toxicity (LD50 >10,000 mg/kg oral in rats; >20,000 mg/kg dermal in rabbits) (Sigma-Aldrich, n.d.)
Skin Sensitization: No sensitization reactions observed in guinea pig studies or murine local lymph node assays; human repeat insult patch tests (HRIPT) showed no sensitization (RIFM assessments cited in ScienceDirect, n.d.)
Irritation: Generally non-irritating at typical use concentrations; high concentrations may cause mild irritation
Genotoxicity: Not genotoxic in standard bacterial reverse mutation assays (Ames test) (Opdyke, 1975)
Phototoxicity: No evidence of phototoxic potential
Environmental Fate: Biodegradable; low aquatic toxicity
Key Safety Considerations:
Highly flammable liquid (Flash Point: 89°C)
Store away from heat, sparks, and open flames
Use in well-ventilated areas
Avoid prolonged or repeated skin contact at high concentrations
References
Arctander, S. (1994). Perfume and flavor materials of natural origin. Allured Publishing Corporation.
Chemicalbook. (2024). Isobornyl acetate. Retrieved from https://www.chemicalbook.com
COSMILE Europe. (2023). Isobornyl acetate – Ingredient. Retrieved from https://cosmileeurope.eu/inci/detail/7360/isobornyl-acetate/
European Chemicals Agency (ECHA). (n.d.). Registration dossier: Isobornyl acetate. Retrieved from https://echa.europa.eu
Flavor and Extract Manufacturers Association (FEMA). (n.d.). Isobornyl acetate – FEMA 2160. Retrieved from https://www.femaflavor.org
International Fragrance Association (IFRA). (2023). Notification of the 51st Amendment to the IFRA Standards. Retrieved from https://ifrafragrance.org
National Institute of Standards and Technology (NIST). (n.d.). Isobornyl acetate. Retrieved from https://webbook.nist.gov
Ning, C., et al. (2012). Study on the catalyst for the synthesis of isobornyl acetate. Industrial Catalysis.
Opdyke, D. L. J. (1975). Monographs on fragrance raw materials: Isobornyl acetate. Food & Cosmetics Toxicology, 13(5), 552-552.
PubChem. (n.d.). Isobornyl acetate, CID 637531. National Center for Biotechnology Information. Retrieved from https://pubchem.ncbi.nlm.nih.gov
Pybus, D., & Sell, C. (Eds.). (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Royal Society of Chemistry.
Scentspiracy. (2024). Isobornyl Acetate (125-12-2) – Premium fresh synthetic ingredient for perfumery. Retrieved from https://www.scentspiracy.com/fragrance-ingredients/p/isobornyl-acetate
ScienceDirect. (n.d.). Bornyl acetate – An overview. Retrieved from https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/bornyl-acetate
Sigma-Aldrich. (n.d.). Isobornyl acetate product information. Retrieved from https://www.sigmaaldrich.com
Wikipedia. (2025). Isobornyl acetate. Retrieved from https://en.wikipedia.org/wiki/Isobornyl_acetate
Sources
Arctander, S. (1961). Perfume and Flavor Materials of Natural Origin
Burdock, G.A. (2010). Fenaroli’s Handbook of Flavor Ingredients, 6th ed.
ChemicalBook – Isobornyl Acetate Properties and Safety Overview
PubChem Compound Summary – CID 11558
FEMA GRAS Database – 2135
Scentspiracy Archives and Technical Database