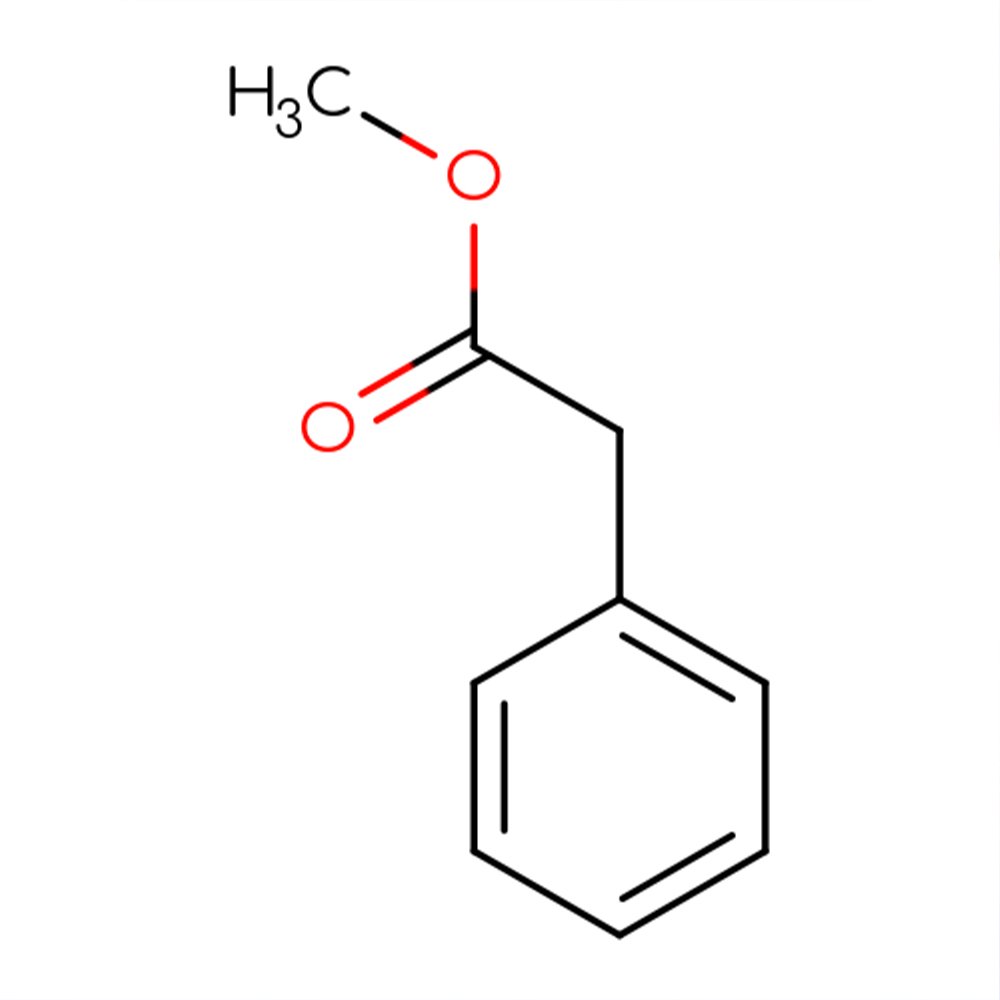
Ethyl Phenylacetate - Technical Ingredient Overview
🔎 Chemical Name — Ethyl 2-phenylacetate (Ethyl benzeneacetate)
🧪 Synonyms — Ethyl phenacetate, Ethyl α-toluate, α-Toluic acid ethyl ester, Phenylacetic acid ethyl ester
📂 CAS Number — 101-97-3
📘 FEMA Number — 2452 (Generally Recognized As Safe for food use)
⚖️ Molecular Weight — 164.20 g/mol
📝 Odor Type — Sweet, floral, honey-like
📈 Odor Strength — Strong to very strong, powerful and diffusive
👃🏼 Odor Profile — Intensely sweet with pronounced honey character; rich floral notes reminiscent of rose, jasmine, and orange blossom; subtle cocoa and balsamic undertones; slightly waxy and animalic nuances in higher concentrations; persistent and tenacious
⚗️ Uses — Perfumery (floral compositions, oriental bases), flavor applications (honey, peach, apricot notes), food and beverage industry, cosmetics and personal care
🧴 Appearance — Clear, colorless to pale yellow liquid
What is Ethyl Phenylacetate?
Ethyl phenylacetate is a synthetic aromatic ester formed through the esterification of phenylacetic acid with ethanol. With the molecular formula C₁₀H₁₂O₂, this colorless to pale yellow liquid possesses an intensely sweet, honey-like fragrance with pronounced floral character. The compound represents one of the phenylacetate ester family, where the acid moiety predominantly determines the overall odor character—a defining feature of aromatic and araliphatic acid esters (Bauer et al., 2008).
While classified as synthetic for commercial purposes, ethyl phenylacetate occurs naturally in trace amounts as a volatile aroma component in various fruits (including apples, figs, guava, pineapple, papaya), honey, wine, cognac, cider, and grape brandy. This natural occurrence contributes to the authentic honey and fruity notes found in these foods and beverages. However, the commercial material is produced synthetically due to cost-effectiveness and consistent quality control.
The synthesis typically proceeds through standard esterification methods: heating phenylacetic acid with ethanol in the presence of an acid catalyst (such as sulfuric acid or hydrochloric acid). Alternative synthetic routes include heating phenylacetonitrile with sulfuric acid in alcohol solution. The resulting ester is then purified through washing with saturated sodium carbonate solution, calcium chloride solution, and sodium chloride solution, followed by distillation under reduced pressure (Bauer et al., 2008).
Historical Background
The history of ethyl phenylacetate is intrinsically linked to the development of synthetic fragrance chemistry in the late 19th and early 20th centuries. As perfumery transitioned from exclusive reliance on natural materials to incorporating synthetic compounds, aromatic esters emerged as valuable tools for perfumers seeking to recreate and enhance floral and fruity notes.
Phenylacetic acid, the parent acid of ethyl phenylacetate, was recognized early in fragrance history for its intense honey-like odor. The compound occurs naturally in neroli oil, Japanese peppermint oil, and trace amounts in rose oils, making it familiar to perfumers working with natural materials. However, phenylacetic acid's extreme potency meant that direct use required extraordinary dilution. The development of phenylacetic acid esters, including the ethyl ester, provided perfumers with more manageable materials that retained the characteristic honey sweetness while offering improved handling properties and broader application versatility.
Throughout the 20th century, ethyl phenylacetate established itself as a workhorse ingredient in both fine and functional perfumery. Its powerful honey-floral character made it particularly valuable in the creation of muguet (lily of the valley), rose, jasmine, sweet pea, and orange blossom compositions. The compound also found extensive application in oriental bases, where its sweet, slightly animalic undertones contributed depth and richness to complex accords. In the flavor industry, recognition of ethyl phenylacetate as a key aroma compound in honey and various fruits led to its approval as FEMA GRAS #2452, establishing its safety for food use (Bauer et al., 2008).
Olfactory Profile
Scent Family: Floral, Honey, Balsamic
Main Descriptors: Powerfully sweet with dominant honey character; rich floral notes suggesting rose, jasmine, and orange blossom; subtle cocoa and balsamic nuances; slightly waxy texture; mild animalic undertones in higher concentrations; persistent sweet-fruity impression reminiscent of apricot and peach
Intensity: Very strong to powerful odor strength with exceptional diffusion and radiance. Highly impactful even in trace amounts (0.01-1% in finished compositions); requires careful dosing to avoid overpowering other ingredients
Tenacity: Excellent longevity for an aromatic ester, displaying good persistence in both top-to-heart note range. The honey-sweet character remains perceptible for several hours on skin and considerably longer on textiles. More tenacious than many simple esters due to its aromatic character
Volatility: Moderate volatility (boiling point ~227°C at standard pressure), functioning primarily as a heart note material with strong initial diffusion. Less volatile than aliphatic esters of comparable molecular weight due to the aromatic ring structure
Fixative Role: While not a traditional fixative like resins or musks, ethyl phenylacetate demonstrates modest substantivity that helps extend and enrich lighter floral notes. Its honey-sweet character provides a unifying effect in complex compositions, helping to blend diverse ingredients into cohesive accords. The compound works particularly well at rounding and sweetening sharp or green notes
Note on Concentration Effects: At very low concentrations (trace amounts), ethyl phenylacetate imparts pleasant honey nuances and subtle floral sweetness. At moderate concentrations (0.1-0.5%), it delivers its characteristic rose-honey profile. At higher concentrations (above 1%), combined with materials like lower alkyl cinnamates, it creates the distinctive "low-cost oriental" note recognizable in many mass-market fragrances. Careful dosing is essential to achieve the desired effect (Arctander, 1960; Bauer et al., 2008).
Applications in Fine Fragrance
Ethyl phenylacetate serves versatile functions across multiple fragrance categories:
Floral Compositions: Core ingredient for creating honey-sweet floral accords; particularly effective in muguet (lily of the valley), rose, jasmine, sweet pea, orange blossom, and lily compositions
Oriental Bases: Provides sweet, slightly animalic depth to oriental fragrances; contributes waxy-balsamic richness; combines effectively with cinnamates for classic oriental character
Functional Fragrance: Widely used in soap perfumes, cosmetics, detergents, and household products where its powerful, cost-effective sweetness provides broad consumer appeal
Modifier & Sweetener: Functions as a powerful sweetening agent for harsh or green notes; helps blend diverse ingredients; adds natural honey character to synthetic compositions
Trace Enhancement: In minute quantities, provides subtle nuances and complexity to sophisticated fine fragrances without dominating the composition
Pairing Behavior: Ethyl phenylacetate blends harmoniously with phenethyl alcohol, benzyl acetate, linalool, geraniol, ionones, coumarin, cinnamates (particularly methyl cinnamate), vanillin, heliotropin, and synthetic musks. It works particularly well with rosy materials, enhancing their natural honey-like facets. The compound also complements citrus notes, adding depth and sweetness to cologne-type fragrances.
Performance in Formula
In fragrance formulations, ethyl phenylacetate requires careful handling due to its high impact:
Typical Usage Levels: 0.01-1% in fine fragrance, 0.1-3% in functional fragrances, up to 8.1 mg/kg (0.00081%) in food applications per FEMA guidelines
Solubility: Insoluble in water; miscible with ethanol, ether, benzene, chloroform, and other organic solvents; readily soluble in alcohol-based perfume concentrates
Color Contribution: Colorless to pale yellow; minimal impact on final product color in typical usage concentrations
Stability: Relatively stable ester that resists hydrolysis under normal storage conditions; should be protected from strong acids or bases which may cause ester cleavage
Technical Considerations: The compound's extreme potency demands precision in dosing—even slight overdosing can result in cloying, unbalanced sweetness. When formulating, perfumers should start with minimal amounts and increase gradually while monitoring the overall balance. The material's tendency to dominate in higher concentrations makes it more suitable for supporting roles in fine fragrance, though it can be featured prominently in cost-effective functional fragrances. Quality control is essential, as impurities or degradation products can introduce off-notes (Arctander, 1960).
Industrial & Technical Uses
Beyond traditional perfumery, ethyl phenylacetate finds application in:
Flavor Industry: Approved food additive (FEMA 2452) used to create honey, peach, apricot, and general fruity-sweet notes in confections, baked goods, beverages, and tobacco flavoring
Wine & Spirits: Recognized as a key aroma compound contributing to honey-like character in wines; monitored in wine analysis as its concentration affects perceived quality (desirable at low levels, considered a defect at high concentrations)
Cosmetic Products: Added to creams, lotions, and personal care items for fragrance and mild sweetening effect
Research Applications: Used as a "greener" solvent alternative in some applications due to its relatively low toxicity and non-mutagenic profile
Pharmaceutical Intermediates: Serves as a precursor in the synthesis of certain pharmaceutical compounds
Regulatory & Safety Overview
IFRA Status: Ethyl phenylacetate is not prohibited or restricted under current IFRA Standards (51st Amendment). The material can be used in fragrance compositions without specific concentration limitations, though formulators should follow general good manufacturing practices and ensure compliance with finished product requirements.
IFRA Documentation: Check current IFRA Standards Library at https://ifrafragrance.org/standards-library for any updates
EU Cosmetics Regulation: Permitted for use in cosmetic products under EU Regulation 1223/2009. Not listed among the 26 declarable allergens requiring specific labeling. Suitable for use in cosmetics when following good manufacturing practices and appropriate concentration guidelines.
FEMA Status: FEMA #2452 - Generally Recognized As Safe (GRAS) for use as a flavoring ingredient under conditions of intended use. Maximum recommended usage levels vary by food category (e.g., 8.1 mg/kg in candy, with appropriate limits for other applications as specified in FEMA guidance and JECFA specifications).
Safety Profile: Ethyl phenylacetate demonstrates a favorable safety profile for cosmetic and flavor applications:
Toxicity: Moderately toxic by ingestion in pure form; safe at appropriate use concentrations
Mutagenicity: Non-mutagenic based on standard testing
Skin Sensitization: Low sensitization potential; generally well-tolerated in cosmetic applications
Environmental: Combustible liquid (flash point ~98.9°C/210°F closed cup); standard precautions for flammable materials apply
Handling: Proper ventilation recommended; avoid inhalation of concentrated vapors; standard chemical safety protocols apply
The compound's long history of safe use in perfumery, cosmetics, and food flavoring, combined with its GRAS status and favorable toxicological profile, supports its continued use across multiple applications when handled appropriately (Bauer et al., 2008).
References
Arctander, S. (1960). Perfume and flavor materials of natural origin. Elizabeth, NJ: Published by the author.
Bauer, K., Garbe, D., & Surburg, H. (2008). Common fragrance and flavor materials: Preparation, properties and uses(5th ed.). Weinheim: Wiley-VCH.
Flavor and Extract Manufacturers Association. (n.d.). FEMA GRAS assessment: Ethyl phenylacetate. Retrieved from https://www.femaflavor.org/flavor-library
Tat, L., Comuzzo, P., Stolfo, I., & Battistutta, F. (2007). Sweet-like off-flavor in Aglianico del Vulture wine: Ethyl phenylacetate as the mainly involved compound. Journal of Agricultural and Food Chemistry, 55(13), 5205-5212. https://doi.org/10.1021/jf0702064
International Fragrance Association. (2023). IFRA Standards Library (51st Amendment). Retrieved from https://ifrafragrance.org/standards-library
European Commission. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Official Journal of the European Union.
Sekar, B. S., Fülöp, F., & Poppe, L. (2022). Bioproduction of natural phenethyl acetate, phenylacetic acid, ethyl phenylacetate, and phenethyl phenylacetate from renewable feedstock. ChemSusChem, 15(5), e202102645. https://doi.org/10.1002/cssc.202102645