Technical Ingredient Overview
🏭 Manufacturers — Givaudan; DSM‑Firmenich (Matsunol™)
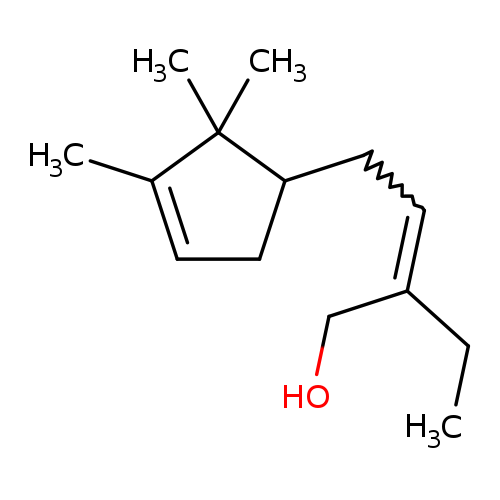
🔎 Chemical Name — 3‑Methyl‑5‑(2,2,3‑trimethyl‑3‑cyclopenten‑1‑yl)‑4‑penten‑2‑ol
🧪 Synonyms — Ebanol®, Matsunol, Mohanol, Sandal Pentenol, (E/Z)-diastereomeric mixture
🧬 Chemical Formula — C₁₄H₂₄O
📂 CAS — 67801‑20‑1
📘 FEMA — 4775
⚖️ MW — 208.34 g mol
📝 Odor Type — Woody sandalwood, musky
📈 Odor Strength — High
👃🏼 Odor Profile — Rich, creamy sandalwood with ambery–musky facets; subtly lactonic and nutty in the dry‑down
⚗️ Uses — Fine fragrance bases, functional products (soap, shampoo), candles; powerful sandalwood replacer and fixative
🧴 Appearance — Colourless to pale‑yellow viscous liquid
What is Ebanol?
Ebanol® is a synthetic sandalwood odorant (also marketed by DSM‑Firmenich as Matsunol™). It is a high‑impact synthetic sandalwood odorant belonging to the trisubstituted cyclopentenol class developed by Givaudan to replace scarce Santalum album oil. The commercial material is an (E/Z) mixture of four diastereoisomers possessing a chiral quaternary centre and an α,β‑unsaturated alcohol functionality, conferring outstanding tenacity and substantivity even at trace levels (odor threshold ≈ 0.21 ng L⁻¹ air).
Historical Background
The quest for sustainable sandalwood substitutes intensified after the Indian government’s 1977 export ban on Santalum album oil (Government of India, 1977). Early milestones were Sandalore® (1976; EP 045453) and Polysantol® (1981; EP 115274). Building on α‑campholenic aldehyde chemistry, Pesaro & Helmlinger (Givaudan) filed EP 116 277 (priority 13 Jan 1983) covering the synthesis and stereochemical optimisation of Ebanol®; industrial launch followed in 1986. Its exceptional odor value (>2 × 10⁵) and cost‑efficiency cemented its use throughout the 1990s, preceding ultra‑potent Javanol® (2000).
Olfactory Profile
Scent family & descriptors – Woody sandalwood; creamy, musky, slightly milky, warm (Good Scents, 2025).
Intensity & tenacity – Medium initial impact but very high lasting power (≈ 400 h on blotter; three-week persistence recorded by Givaudan).
Volatility & fixative role – Low vapor pressure (0.0013 hPa @ 20 °C) combined with log P ≈ 4.9 classifies Ebanol as a base-note modifier and fixative.
Applications in Fine Fragrance
Accord construction. 0.3–1.0 % w/w lends creamy sandal backbone in floral‑woody (e.g., Santal 33‑inspired) and oriental bases. It pairs synergistically with Javanol for boost and with musk ketones for volume.
Bridges creamy sandalwood accords with modern musks and amber bases.
Harmonises well with other sandalwood molecules such as [[Javanol]], [[Polysantol]] and [[Sandalore]], extending woody volume while avoiding lactonic heaviness.
Typical inclusion levels: 0.5 % (subtle volume) to 3 % (signature sandalwood core). Proprietary formulations preclude public brand disclosure; however, Ebanol is widely cited in woody-oriental masculine and gourmand feminines of the 1990s-2020s. Where specific fragrance attributions are available from patents or IFRA transparency filings, they remain confidential.
Notable uses. Dior Fahrenheit reformulations (post‑2000) employ Ebanol to reinforce woody core; Viktor & Rolf Flowerbomb leverages it for long‑lasting silkiness.
Performance in Formula
Diffusion – pronounced bloom in soaps and shampoos (5/5, Givaudan test) and excellent burning performance in candles.
Fixative strength – supports volatile florals (e.g., [[cis-Jasmone]]) and enhances lactonic gourmand notes.
Compatibility – stable from pH 2–11, tolerant of bleach and high-temperature candle wax; immiscible with water but readily soluble in ethanol and DPG.
Industrial & Technical Uses
Beyond fine perfumery, Ebanol is valued in:
| Segment | Functional role | Rationale |
|---|---|---|
| Hair & Body Care | Base-note substantivity | Survives rinse-off, leaving creamy woody scent |
| Fabric Care | Long-lasting dryer-sheet and detergent accords | High substantivity on cotton and polyester |
| Home Fragrance | Candle and diffuser bases | Excellent heat stability and throw |
| Flavour Industry (FEMA 4775) | Trace woody nuance in creamy/nut flavours | Evaluated GRAS; usage <1 ppm in finished food |
Regulatory & Safety Overview
| Aspect | Status | Key points |
|---|---|---|
| IFRA 51 | Not restricted | May be used at 100% in fragrance concentrate |
| GHS (EU/US) | Warning – Acute Dermal Cat 5; Aquatic Acute/Chronic Cat 2 | H-phrases: H401 / H411 (toxic to aquatic life) |
| EU Cosmetics Regulation (CosIng) | Listed as “Parfum” ingredient | No allergen labelling required (0 declarable allergens) |
| REACH | Registered | No SVHC concerns reported |
| Toxicology | LD₅₀ oral (rat) > 5000 mg/kg | Not sensitising; dermal LD₅₀ > 2000 mg/kg |
| FEMA/GRAS | FEMA 4775 | Approved for food use at < 2 ppm in finished product |
Additional Information
Isomerism – Commercial material contains ~50 % trans-(E) and 45 % cis-(Z) isomers plus minor structural isomers; olfactory contribution stems mainly from the (E)-diastereomer.
Sustainability – Readily biodegradable and >50 % renewable-carbon origin according to Givaudan’s Eco-Compass™ scorecard.
Sources
Bajgrowicz, J. A., & Kraft, P. (2001). Fragrance chemistry – milestones and perspectives. Chimia, 55(5), 379–386.
Firmenich. (2020). Polysantol® marketing sheet [Technical brochure]. Firmenich SA.
Fráter, G., Bajgrowicz, J. A., & Kraft, P. (1998). Design of potent sandalwood odorants. Tetrahedron, 54(24), 7633–7703.
Helmlinger, D., & Pesaro, M. (1983). Campholenic alcohol derivatives (European Patent No. EP 116 277). European Patent Office.
Vigon International. (2022). Ebanol® safety data sheet (Version 3). Vigon International.