Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Dimethyl Acetaldehyde Phenyl Acetal (PADMA)
Premium Synthetic Ingredient for Perfumery
Dimethyl Acetaldehyde Phenyl Acetate (commonly referred to as PADMA) is a synthetic green-floral material derived from phenylacetaldehyde. It delivers leafy, lilac-like floral notes with superior oxidative stability. Its primary role in perfumery is as a green-floral modifier and stabilizing surrogate for aldehydes. It is widely used in hyacinth, lilac, rose, and fougère-type formulations.
Also approved as a FEMA flavoring at trace levels.
Premium Synthetic Ingredient for Perfumery
Dimethyl Acetaldehyde Phenyl Acetate (commonly referred to as PADMA) is a synthetic green-floral material derived from phenylacetaldehyde. It delivers leafy, lilac-like floral notes with superior oxidative stability. Its primary role in perfumery is as a green-floral modifier and stabilizing surrogate for aldehydes. It is widely used in hyacinth, lilac, rose, and fougère-type formulations.
Also approved as a FEMA flavoring at trace levels.
Premium Synthetic Ingredient for Perfumery
Dimethyl Acetaldehyde Phenyl Acetate (commonly referred to as PADMA) is a synthetic green-floral material derived from phenylacetaldehyde. It delivers leafy, lilac-like floral notes with superior oxidative stability. Its primary role in perfumery is as a green-floral modifier and stabilizing surrogate for aldehydes. It is widely used in hyacinth, lilac, rose, and fougère-type formulations.
Also approved as a FEMA flavoring at trace levels.
Technical Ingredient Overview
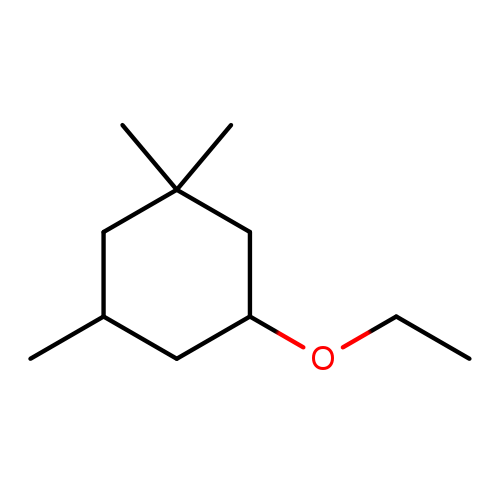
🔎 Chemical Name (INCI) — Phenylacetaldehyde dimethyl acetal
🧪 Synonyms — PADMA, Viridine®, Rosal, Vert de Lilas, Vertodor, Veracetal, Foliacetal, 1,1‑Dimethoxy‑2‑phenylethane, 2,2‑Dimethoxy‑1‑phenylethane
🧬 Chemical Formula — C₁₀H₁₄O₂
📂 CAS — 101‑48‑4
📘 FEMA — 2876
⚖️ MW — 166.22 g/mol
📝 Odor Type — Green-floral (hyacinth, lilac, rose)
📈 Odor Strength — High (Relative Odor Impact ≈ 500; ≈ 25 h on strip)
👃🏼 Odor Profile — Fresh green foliage, earthy plant-stem, hyacinth, lilac, rose, gardenia, honeyed fruity nuances (peach, apricot)
⚗️ Uses — Green-floral modifier in fine fragrance (hyacinth, lilac, muguet, rose, fougère); flavouring ingredient ≤ 10 ppm (FEMA 2876); stabilising surrogate for phenylacetaldehyde
🧴 Appearance — Colourless to pale-yellow clear liquid; density ≈ 1.01 g/cm³; flash-point ≈ 88–90 °C
What is Dimethyl Acetaldehyde Phenyl Acetate (PADMA)?
Phenylacetaldehyde dimethyl acetal, also known commercially as PADMA or Viridine®, is a stable acetal derived from phenylacetaldehyde. It was developed to overcome the instability and oxidation-proneness of its parent aldehyde, enabling longer-lasting and fresher green floral notes in perfumery. Its acetal structure not only confers oxidative resistance but also introduces nuanced leafy and lilac-like facets that are prized in floral reconstructions.
Historical Background
Although an exact date of discovery is not documented, PADMA is believed to have entered the perfumery industry in the early 1950s. Steffen Arctander highlighted it in his Perfume and Flavor Chemicals compendium (1960) as one of the most widely used acetals at the time. It quickly became valued for its ability to deliver hyacinth, lilac, and foliage notes in a stable, non-reactive form. Its historical role was pivotal in the green floral wave of the 1960s and 70s, where it became a backbone ingredient in both soliflores and complex fougères.
Olfactory Profile
Scent Family: Green-floral
Descriptors: Hyacinth, lilac, fresh greenery, rose leaf, gardenia, with subtle mushroomy and metallic undertones at high concentrations.
Intensity & Tenacity: Considered a heart note with strong diffusivity and lasting approximately 25 hours on a scent strip.
Applications in Fine Fragrance:
PADMA is a foundational building block in hyacinth and lilac accords, often combined with cis-3-hexen-1-ol, phenylacetaldehyde, and muguet-type materials. It lends realism to rose, violet, and gardenia bases, and reinforces the “stem” effect in green florals. It is frequently found in chypres, fougères, and muguet-type perfumes.
Performance in Formula:
Highly diffusive, with excellent stability under various pH conditions. It does not discolor and is alcohol- and soap-stable. Its dual role as both a modifier and a fixative makes it invaluable for floral-green top and heart notes.
Industrial & Technical Uses
In addition to its perfumery applications, PADMA (FEMA 2876) is approved for use as a flavouring agent in beverages, liqueurs, and sweets, where it imparts subtle green and fruity-floral tones (e.g., peach, apricot).
Its stability and aldehyde-masking properties make it suitable for technical perfumery in household products, where it can withstand formulation stresses without degradation.
Also used as a pharmaceutical and agrochemical intermediate in fine chemical synthesis.
Regulatory & Safety Overview
| Aspect | Status |
|---|---|
| IFRA | Not restricted under IFRA 51st Amendment (CAS 101-48-4 not listed in Annexes) |
| FEMA GRAS | Approved under FEMA No. 2876 |
| EU Cosmetics Regulation | Not listed in Annex II/III — permitted without restriction |
| GHS Classification | Combustible liquid (H227); general handling precautions recommended |
| Toxicology | Low dermal sensitization; NOAEL 200 mg/kg/day (RIFM, 2022) |
| Environmental | Not considered persistent, bioaccumulative, or toxic (non-PBT); low risk of aquatic toxicity |
| Allergens | Not classified among EU fragrance allergens (26-list) |
Additional Information
Synergistic materials: phenylacetaldehyde, phenyl ethyl alcohol, cis-3-hexenol, hydroxycitronellal, methyl dihydrojasmonate.
Internal cross-links:
Explore its role in Phenylacetaldehyde and compare with cis-3-Hexenol for green-floral accord development.
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Vol. I). Self-published.
ChemicalBook. (2025). Phenylacetaldehyde dimethyl acetal – Properties and Uses.
Fragrance Conservatory. (n.d.). Phenylacetaldehyde Dimethyl Acetal.
IFRA. (2023). IFRA Standards – 51st Amendment. Geneva: International Fragrance Association.
PerfumersWorld. (2024). PADMA Technical Sheet.
RIFM. (2022). Fragrance Ingredient Safety Assessment: Phenylacetaldehyde Dimethyl Acetal. Food and Chemical Toxicology, 169, 113493.
TCI America. (2024). Phenylacetaldehyde Dimethyl Acetal Safety Data Sheet.