Technical Ingredient Overview
🏭 Manufacturer — Symrise AG
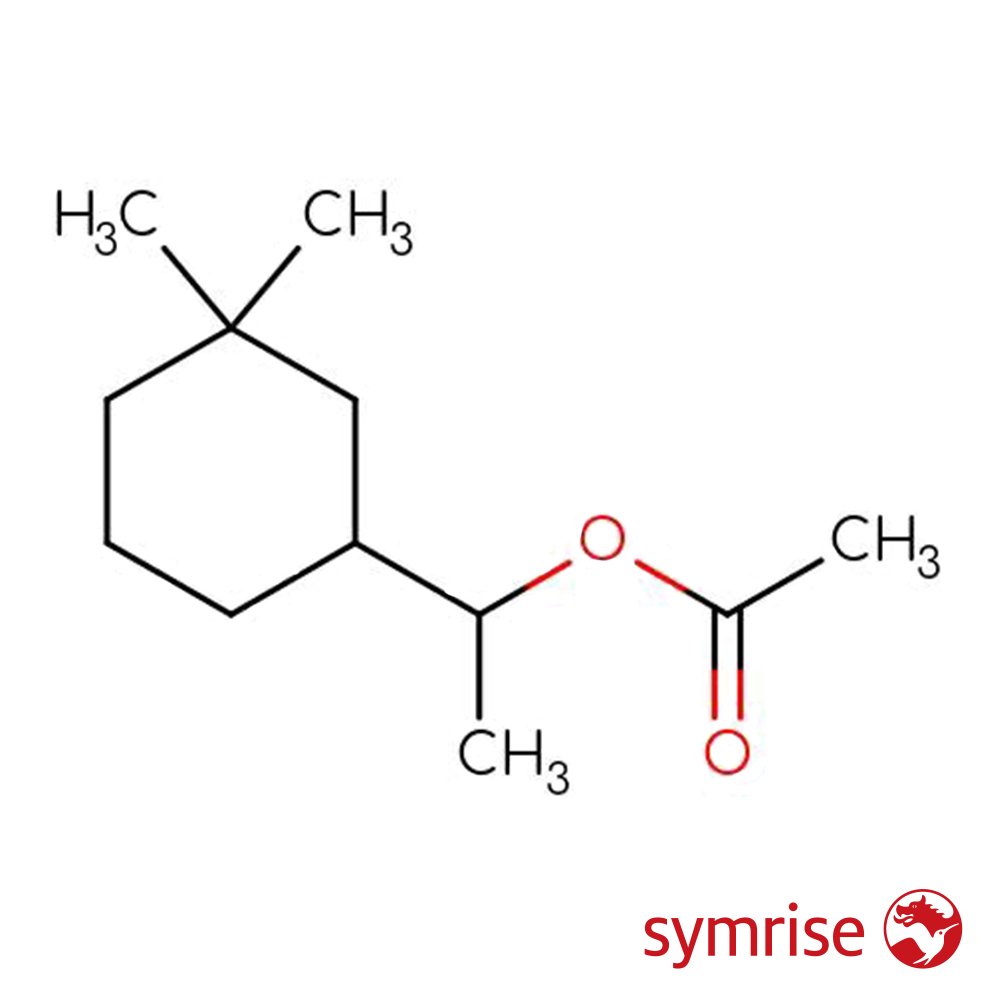
🔎 Chemical Name — 1-(3,3-Dimethylcyclohexyl)ethyl acetate; α,3,3-Trimethylcyclohexylmethyl acetate
🧪 Synonyms — Musk Acetate; Rosamusk; Cyclohexane-1-methanol, α,3,3-trimethyl, acetate; Terpene ester (proprietary designation)
📂 CAS Number — 25225-10-9
📘 FEMA Number — Not listed (not approved for flavor applications)
⚖️ Molecular Weight — 198.30 g/mol
🧬 Molecular Formula — C₁₂H₂₂O₂
📝 Odor Type — Floral-fruity with musky undertones
📈 Odor Strength — Medium (moderate diffusive power)
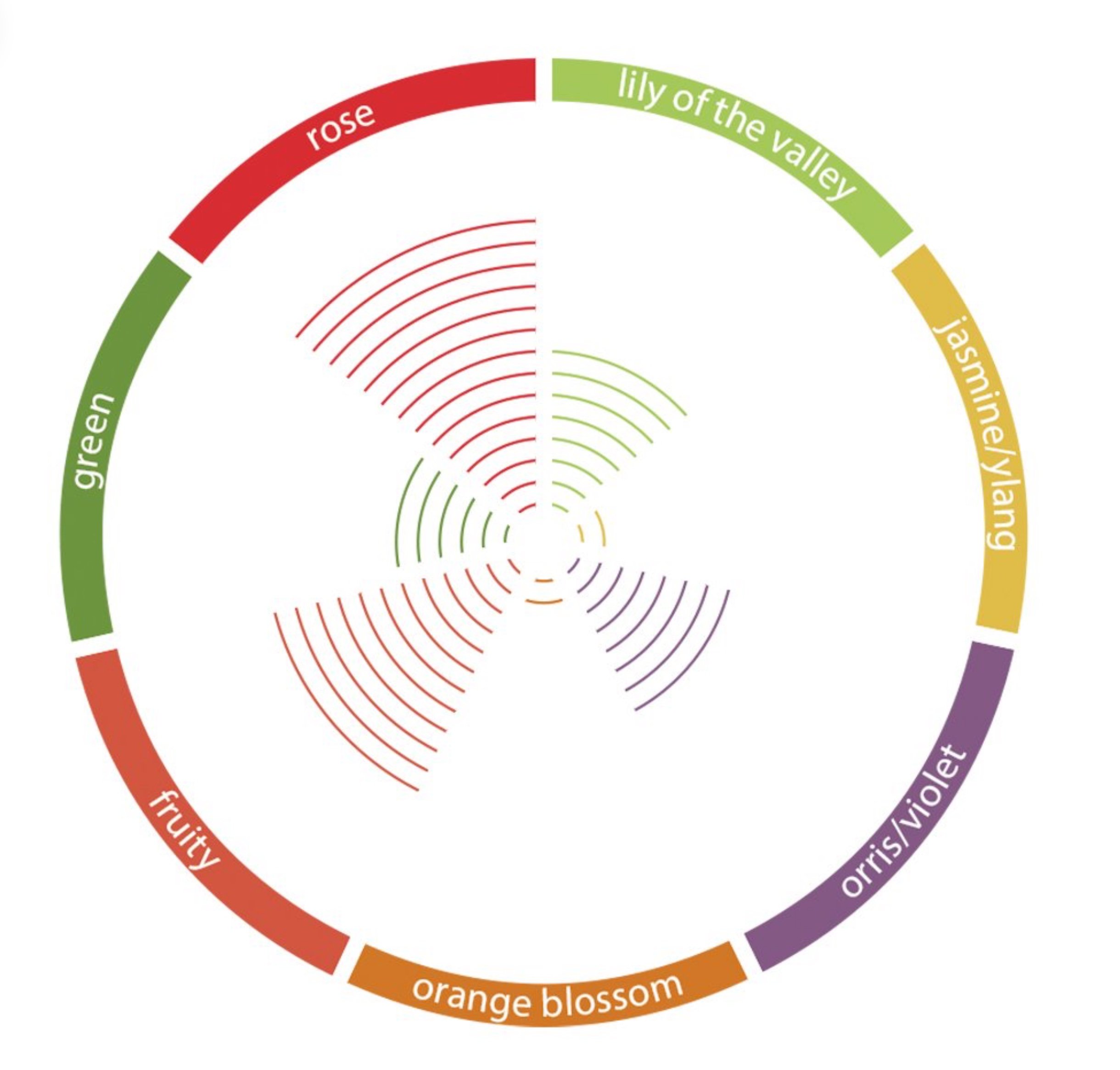
👃🏼 Odor Profile — Floral-rose, geranium leaf, damascone-like → Fruity-pear, orris-like, clean musky → Herbal-green, powdery, ambery-woody base → Soft, diffusive, volume-building with subtle complexity
⚗️ Uses — Floral accord enhancement and volume building, Diffusion booster in top and middle notes, Green-herbal harmonizer, Musky-fruity modifier in fine fragrance, Functional fragrance applications (soaps, detergents, personal care). Recommended usage level: 0.5–8% in final composition
🧴 Appearance — Clear, colorless to pale yellow liquid
🌡️ Boiling Point — 132°C (at standard atmospheric pressure)
🧪 GC Purity — ≥90% (sum of four stereoisomeric forms)
♻️ Sustainability Profile — 83% renewable carbon content; 66% ready biodegradability (OECD 301 guidelines)
What is Cyclodumol Acetate?
Cyclodumol Acetate is a synthetic terpene-derived ester belonging to the class of cycloaliphatic acetates used extensively in modern perfumery for their floral-fruity-musky characteristics and sustainability profile. The compound is structurally characterized by a substituted cyclohexane ring bearing a tertiary methyl group and an acetylated secondary alcohol, resulting in a mixture of stereoisomers that contribute to its complex olfactory signature (Symrise, 2022).
Chemically classified as an alicyclic ester, Cyclodumol Acetate represents a new generation of bio-based fragrance materials derived from crude sulfate turpentine (CST), a renewable byproduct stream of the kraft pulping process used in paper manufacturing. This positions the ingredient within the broader industry movement toward circular economy principles and reduced reliance on petrochemical feedstocks.
The material is produced through a multi-step synthetic pathway involving terpene isolation, selective hydrogenation, and acetylation, yielding a product with reproducible olfactive properties and high chemical stability. Its designation as "Cyclodumol Acetate" is a proprietary trade name registered by Symrise AG, while the compound is also referenced in technical literature as "Musk Acetate" or "Rosamusk" by various suppliers.
Historical Background
Cyclodumol Acetate was officially introduced to the fragrance industry at the SIMPPAR 2022 Conference(Symposium on Innovation in Materials for Perfumery and Aroma Research) held in Marseille, France, as part of Symrise's broader commitment to sustainable raw material innovation (Perfumer & Flavorist, 2022). The development timeline reflects approximately five years of research and optimization focused on converting industrial waste streams into high-performance fragrance ingredients.
The compound emerged from Symrise's "Renewable Carbon Platform," an initiative launched in the late 2010s to develop fragrance materials with measurable environmental benefits without compromising olfactive quality or formulation performance. This program specifically targeted crude sulfate turpentine (CST) from the southeastern United States pine forests, where sustainable forestry practices and advanced pulp processing technologies provide consistent feedstock availability.
The first commercial batches became available in early 2022, with full-scale production established by mid-2023. Since its introduction, Cyclodumol Acetate has gained recognition for its dual value proposition: ecological responsibility combined with functional versatility as both a cost-effective volume builder and a sophisticated floral modifier.
The compound's development represents a strategic response to multiple industry pressures:
Growing regulatory scrutiny of synthetic musks and petrochemical-derived materials
Consumer demand for "clean" and sustainable beauty products
Economic drivers favoring materials that can function at both low and high dosages
Technical requirements for biodegradable ingredients compatible with modern detergent systems
Unlike many historical fragrance innovations driven purely by novel olfactive character, Cyclodumol Acetate was engineered from its inception to meet contemporary sustainability standards while delivering reliable performance across multiple fragrance categories.
Olfactory Profile
Scent Family: Floral-Fruity with Musky-Woody undertones; categorized within the broader class of soft musks and floral volume builders
Main Descriptors:
Floral: Dominant rose-geranium character with orris-like powdery facets; reminiscent of damascone beta but softer and less ionone-sharp
Fruity: Distinct pear-apple nuances with subtle tropical-peach undertones; contributes fresh, juicy topnotes without excessive sweetness
Musky: Clean, soft musk character providing warmth and diffusion; lacks the animalic or heavy aspects of traditional musk compounds
Herbal-Green: Secondary green-leaf and herbal-geranium notes that add complexity and natural freshness
Powdery-Ambery: Base notes reveal subtle powder and pale wood characteristics that enhance longevity
Intensity: Medium odor strength (rated 4-5 on a 10-point scale). The material exhibits good initial impact without being overpowering, making it suitable for both accent and volume applications. At recommended usage levels (0.5-8%), it provides noticeable floral lift without dominating the composition.
Tenacity: Moderate longevity with primary olfactive presence lasting 4-6 hours on blotter at 10% dilution in DPG. The material exhibits a relatively smooth dry-down that transitions from bright floral-fruity topnotes to softer musky-woody base notes. Fixative properties are moderate; while not a true fixative, it does contribute to overall composition stability and helps bridge volatile top notes with heavier base materials.
Volatility:
Classification: Middle-to-top note material with good diffusion characteristics
Evaporation Rate: Relatively volatile for an ester of its molecular weight (MW 198.30 g/mol); boiling point of 132°C indicates moderate vapor pressure
Note Distribution: Primary impact in top-middle notes (first 30-90 minutes), with sustained presence extending into heart notes (2-4 hours)
Diffusion: Exhibits good radiance and diffusive power, particularly effective in enhancing the projection of floral compositions without overwhelming delicate nuances
Fixative Role: Cyclodumol Acetate functions primarily as a floral volume builder and diffusion enhancer rather than a classical fixative. Its role in fragrance architecture includes:
Amplifying the presence and perceived intensity of floral accords (rose, geranium, violet, iris)
Bridging the gap between highly volatile citrus-aldehydic top notes and less volatile floral middle notes
Providing a soft musky halo that unifies disparate olfactive elements
Contributing to "fullness" or "body" in compositions that might otherwise feel thin or sharp
Moderately slowing the evaporation of accompanying volatile materials through vapor pressure effects
The material's ester functionality provides inherent stability in most fragrance bases, though it may undergo slow hydrolysis in highly acidic or alkaline conditions over extended storage periods.
Applications in Fine Fragrance
Cyclodumol Acetate has established itself as a versatile tool in modern perfumery, valued for its ability to enhance floral compositions while maintaining a contemporary, clean aesthetic. Its primary applications include:
Role in Fragrance Compositions:
Floral Volume Builder: Adds perceived "body" and fullness to rose, geranium, muguet, and peony accords without contributing excessive weight or muddiness
Diffusion Enhancer: Improves the radiance and projection of fragrance formulas, particularly in alcohol-based fine fragrances and body mists
Fruity-Floral Bridge: Connects bright fruity top notes (pear, apple, berries) with deeper floral heart notes, creating seamless transitions
Green-Floral Modifier: Softens sharp or overly herbaceous green notes (galbanum, violet leaf, fig) while preserving freshness
Musky Background: Provides clean, modern musk character that supports contemporary "skin scent" and "fresh laundry" aesthetics
Typical Accords and Combinations:
Rose Accords: Pairs exceptionally well with phenylethyl alcohol, geraniol, citronellol, and damascones to create natural, multi-dimensional rose effects
Geranium Compositions: Complements geranium bourbon and geranium Egypt oils, adding fruity-pear nuances that soften the minty-green aspects
Pear Notes: Works synergistically with pear esters (ethyl hexanoate, hexyl acetate) and damascones to create realistic pear effects
Fruity-Floral Blends: Combines effectively with strawberry, raspberry, and peach modifiers (gamma-undecalactone, furaneol derivatives) in "fruit blossom" themes
Soft Musk Bases: Blends seamlessly with modern synthetic musks (galaxolide, celestolide, exaltolide) to create clean, diffusive musk foundations
Performance in Formula
Behavior in Blends and Mixtures: Cyclodumol Acetate demonstrates predictable and stable behavior across diverse fragrance systems. As an ester, it exhibits good chemical stability under normal formulation and storage conditions, with minimal tendency toward oxidation, polymerization, or degradation. The material integrates smoothly into both simple and complex accords without causing phase separation, precipitation, or color changes.
In multi-component floral formulas, Cyclodumol Acetate tends to act as a "unifier," smoothing transitions between disparate olfactive elements and reducing harsh or discordant notes. This behavior is particularly valuable in bridging bright aldehydic or citrus top notes with heavier floral absolutes or woody bases.
Diffusion Characteristics: The material exhibits moderate-to-good diffusive properties relative to its molecular weight and vapor pressure. Its ability to enhance the perceived radiance of a composition stems from both direct olfactive contribution and indirect effects on the evaporation kinetics of neighboring volatiles.
Key diffusion behaviors include:
Vapor-Phase Enhancement: The compound's moderate volatility allows it to lift and carry less volatile floral materials into the headspace, increasing their perceived intensity
Odor Threshold Effects: At concentrations below 1%, Cyclodumol Acetate can function as a "blooming agent," amplifying the impact of trace-level materials without being directly perceptible itself
Temporal Evolution: The material's odor profile evolves smoothly over time, avoiding abrupt character changes that can create discontinuities in fragrance development
Environmental Persistence: In functional applications (laundry, fabric care), the material's biodegradability ensures it does not accumulate in wastewater or persist in the environment, addressing growing regulatory and consumer concerns
Impact on Overall Composition:
Volume and Fullness: Adds perceived "weight" or "body" to compositions without increasing substantivity or creating heaviness
Brightness and Lift: Contributes to the overall "sparkle" or freshness of floral-fruity formulas
Naturality: Despite its synthetic origin, the material can enhance the perceived naturalness of botanical accords, particularly in rose, geranium, and fruit blossom themes
Cost-Efficiency: At higher usage levels (5-8%), can serve as an economical volume builder, reducing reliance on more expensive naturals or specialty materials
Fixative Strength and Properties: While not a traditional fixative in the sense of substantive materials like musks, resins, or heavy woods, Cyclodumol Acetate does contribute to overall composition stability through several mechanisms:
Vapor Pressure Modulation: The material's intermediate volatility can subtly slow the evaporation of more volatile components through vapor-phase interactions
Olfactive Masking: By providing continuous floral-fruity character throughout the dry-down, it masks the gradual loss of top notes and creates an impression of greater longevity
Molecular Interactions: Hydrogen bonding and Van der Waals forces between the acetate group and other functional groups (alcohols, aldehydes) may reduce overall evaporation rates at the molecular level
Compatibility with Other Materials:
Excellent: Aldehydes, alcohols, esters, ethers, citrus oils, floral absolutes, woody materials
Good: Ionones, damascones, phenolic materials, synthetic musks, lactones
Moderate: Highly reactive materials (strong acids, oxidizers); generally stable but may require evaluation in specific systems
Not Recommended: Strong bases or nucleophiles that could cause ester hydrolysis over time
Industrial & Technical Uses
While Cyclodumol Acetate was developed primarily for fine fragrance applications, its technical properties and sustainability profile have expanded its utility into several industrial sectors:
Functional Fragrance Applications:
Laundry Detergents and Fabric Softeners: The material's biodegradability (66% ready biodegradability per OECD 301 standards) makes it particularly valuable in this category, where environmental persistence is a growing regulatory concern. It contributes fresh, floral-fruity character that survives wash cycles and provides pleasant residual scent on fabrics
Personal Care and Cosmetics: Widely used in body washes, shampoos, conditioners, lotions, and creams where its soft floral character aligns with contemporary "clean beauty" positioning
Household Cleaning Products: Applied in surface cleaners, air fresheners, and multi-purpose cleaners where pleasant odor is desired without environmental persistence
Industrial Odor Masking: Employed in low concentrations to mask unpleasant base odors in technical products without contributing strong fragrance character
Non-Fragrance Applications:
Green Chemistry Research: The material serves as a model compound for sustainable fragrance ingredient development, demonstrating that bio-based terpene derivatives can match or exceed the performance of petroleum-derived alternatives
Biodegradability Studies: Used in academic and industrial research on fragrance material environmental fate, particularly in wastewater treatment and aquatic toxicology studies
Renewable Carbon Metrics: Featured in life cycle assessments (LCA) and carbon footprint analyses as a case study for renewable carbon integration in specialty chemicals
Industrial Processes and Functions: The production of Cyclodumol Acetate involves several key industrial processes:
Terpene Extraction: Crude sulfate turpentine (CST) from kraft pulping is distilled and purified to isolate specific terpene fractions
Selective Hydrogenation: Terpene olefins are catalytically hydrogenated under controlled conditions to yield the desired cyclohexane derivatives
Acetylation: The resulting tertiary alcohol is acetylated using acetic anhydride or acetyl chloride in the presence of appropriate catalysts
Purification: Final product is distilled and filtered to achieve ≥90% purity (sum of stereoisomers)
Environmental and Process Safety:
Biodegradability: 66% ready biodegradability (OECD 301 guidelines), indicating the material is readily broken down by environmental microorganisms
Aquatic Toxicity: Not classified as hazardous to aquatic life at typical use concentrations; specific LC50 and EC50 data available in supplier safety documentation
Worker Safety: Standard precautions for organic esters apply; minimal skin or respiratory irritation potential at typical workplace exposures
Waste Disposal: Can be treated via conventional wastewater treatment systems due to biodegradability; excess material should be disposed of according to local regulations for organic chemical waste
Regulatory & Safety Overview
IFRA Status: Cyclodumol Acetate is not restricted or prohibited under the IFRA Standards 51st Amendment (2024).
EU Cosmetics Regulation: Compliant with Regulation (EC) No 1223/2009 governing cosmetic products in the European Union. Cyclodumol Acetate is:
Not listed among the 26 declarable fragrance allergens in Annex III of the Cosmetics Regulation
Not prohibited or restricted under Annexes II, III, or IV
Suitable for use in cosmetic and personal care products without mandatory declaration beyond general "parfum/fragrance" listing
FEMA Status: Not listed in the Flavor and Extract Manufacturers Association (FEMA) GRAS database. Cyclodumol Acetate is not approved for use as a food flavoring agent or for ingestion. The material should not be incorporated into products intended for oral consumption, including flavor applications, oral care products intended to be swallowed, or food contact materials.
Sources
Perfumer & Flavorist. (2022). Symrise's Cyclodumol Acetate. Perfumer & Flavorist Magazine. Retrieved from https://www.perfumerflavorist.com/fragrance/ingredients/news/21875219/symrise-9280-symrises-cyclodumol-acetate
Symrise AG. (2022). SIMPPAR 2022: Sustainable innovation in fragrance materials [Conference presentation]. Symposium on Innovation in Materials for Perfumery and Aroma Research, Marseille, France.
Symrise AG. (2024). Cyclodumol Acetate technical datasheet. Symrise Fragrance Materials Division.
Organisation for Economic Co-operation and Development. (1992). OECD Guidelines for the Testing of Chemicals, Section 3: Test No. 301: Ready Biodegradability. OECD Publishing.
International Fragrance Association. (2024). IFRA Standards 51st Amendment. Retrieved from https://ifrafragrance.org/standards
European Commission. (2009). Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Official Journal of the European Union.
PubChem [Database]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 92196229, Cyclodumol Acetate. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/92196229