Cashmeran® Technical Ingredient Overview
🏭 Manufacturer — International Flavors & Fragrances (IFF)
🔎 Chemical Name — 6,7-Dihydro-1,1,2,3,3-pentamethyl-4(5H)-indanone
🧪 Synonyms — DPMI, 1,2,3,5,6,7-hexahydro-1,1,2,3,3-pentamethyl-4H-inden-4-one; 4H-Inden-4-one, 1,2,3,5,6,7-hexahydro-1,1,2,3,3-pentamethyl-; 1,1,2,3,3-Pentamethyl-2,3,6,7-tetrahydro-1H-indene-4(5H)-one; Musk indanone; Indomuscone; Dihydro pentamethylindanone
📂 CAS Number — 33704-61-9
📘 FEMA Number — Not applicable (fragrance ingredient only)
⚖️ Molecular Weight — 206.32 g/mol
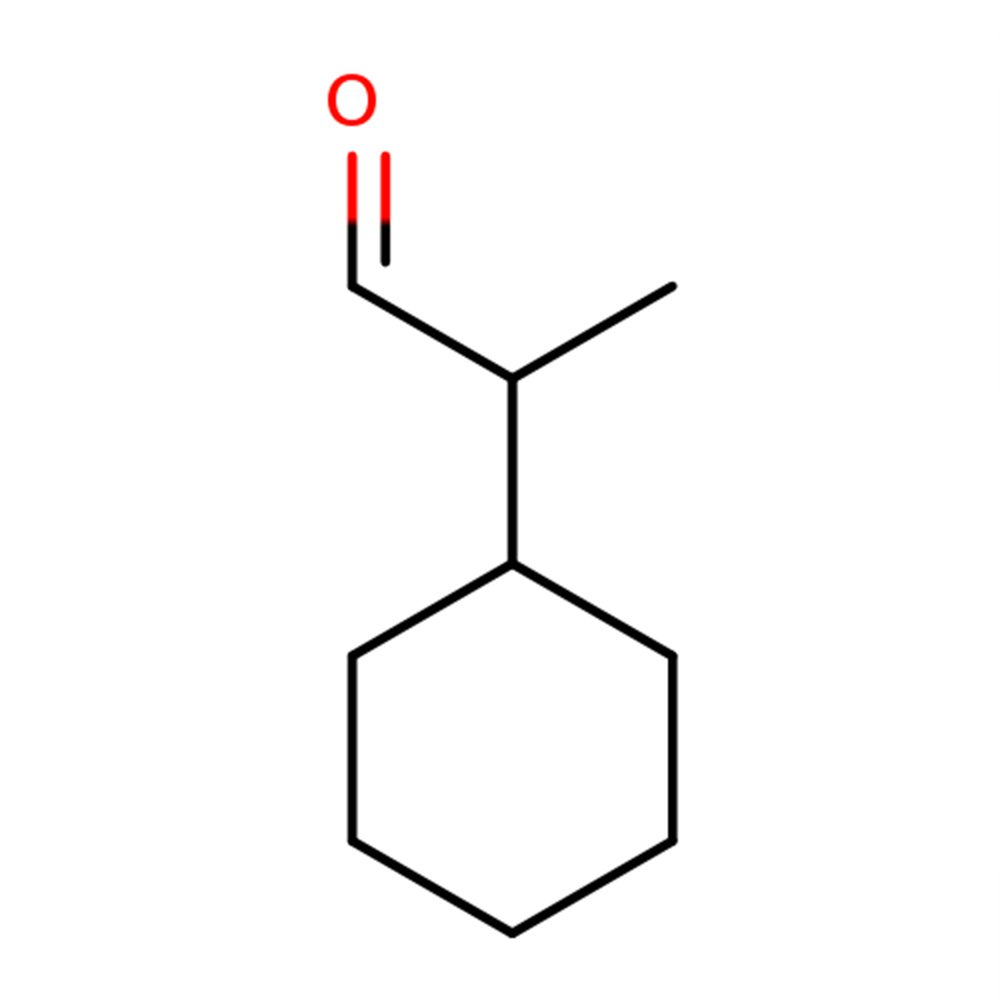
🧬 Molecular Formula — C₁₄H₂₂O
📝 Odor Type — Woody-Musky-Amber
📈 Odor Strength — Strong, diffusive, long-lasting
👃🏼 Odor Profile — Warm, musky, woody with spicy-floral nuances, powdery, velvet-like texture with coniferous undertones. Combines softness of musks with richness of woods and subtle floral spiciness, creating distinctive “cashmere-like” smooth, enveloping sensation
⚗️ Uses — Fine fragrance (woody, oriental, amber accords), functional fragrance, woody-musky depth enhancement, bridge material linking musky softness with woody structure
🧴 Appearance — Pale yellow liquid to white solid (melting point 27°C); density: 0.954-0.962 at 20°C; refractive index: 1.497-1.502 at 20°C
What is Cashmeran®?
Cashmeran® is a synthetic polycyclic ketone belonging to the indanone chemical family. Unlike traditional musk compounds, Cashmeran® represents a unique olfactory bridge between woody and musky notes, characterized by its complex, multi-faceted scent profile (Ohloff et al., 2012). The molecule features an unsaturated bicyclic structure with a ketone functional group that serves as the primary osmophore responsible for its distinctive aroma.
Despite being commonly referenced as a polycyclic musk, Cashmeran® does not technically belong to this category according to IFRA’s official definition. IFRA defines polycyclic musks as compounds with molecular formulas C₁₇H₂₄O or C₁₈H₂₆O containing one central aromatic benzene ring and used primarily for their musk odor (Wikipedia, 2025). Cashmeran®, with its C₁₄H₂₂O formula and distinctive woody-amber character beyond pure musk, represents a separate class of olfactory materials.
The ingredient does not occur naturally and exists as a racemic mixture containing an asymmetric carbon center. While constitutional isomers such as alpha-ionone and isoraldeine share structural similarities, they exhibit entirely different olfactory profiles, demonstrating the critical relationship between molecular structure and scent perception in fragrance chemistry.
Historical Background
Cashmeran® was discovered in 1969 by scientist John B. Hall while working for International Flavors & Fragrances (IFF). The discovery emerged from Hall’s research into cost-effective chemical transformations using pentamethylindane and tetramethylnaphthalene molecular structures. The development was officially documented in U.S. Patent No. 3,773,836, filed on August 18, 1969 (Hall, 1969).
The invention of Cashmeran® represented a significant milestone in synthetic perfumery, as Hall identified this unsaturated ketone as an important new fragrance ingredient during his systematic exploration of indanone derivatives. The molecule’s creation occurred during a period of intense innovation in synthetic musks and woody materials, when the fragrance industry was actively seeking alternatives to natural materials and developing new olfactory dimensions.
Initially introduced to the market in the 1970s, Cashmeran® was designed to bring a novel musky-woody dimension to modern perfumery. According to historical accounts from IFF’s internal documentation, the material was first developed after analysis of an impurity detected through gas chromatography during work on another fragrance compound. Subsequent improvements in production processes have significantly enhanced its commercial viability and expanded its usage possibilities in fine fragrance and functional applications (Kraft et al., 2020).
The discovery coincided with the golden age of synthetic musk development, when fragrance houses were creating entirely new olfactory territories beyond natural extraction capabilities. Cashmeran® quickly established itself as a versatile ingredient that perfumers could employ across multiple fragrance families, from florals to dark woods and oriental accords.
Olfactory Profile
Scent Family
Cashmeran® bridges multiple olfactory families, primarily positioned within the woody-musky-amber spectrum. The IFF compendium classifies it as an amber ingredient rather than primarily as a musk odorant, reflecting its complex character that transcends simple categorization (IFF, 2025).
Main Descriptors
The scent combines rich, spicy, fruity, chypre, balsamic, and vanilla notes with distinctive woody-musky undertones. Descriptors include diffusive, velvet-like, powdery, warm, silky smooth, with notable coniferous-type woody aspects. The olfactory experience presents a unique balance between floral-fruity musky character and conifer-type woody elements, creating a multidimensional sensory impression that distinguishes it from conventional musk or wood ingredients (Kraft & Pickenhagen, in Ohloff et al., 2012).
The material exhibits what fragrance chemist Arcadi Boix Camps described as “a strong, floral, musky product of great diffusion and personality,” noting the difficulty of placing this unique material in any single category. The scent conveys the soft, sensuous feeling of cashmere fabric, which inspired its commercial name (Camps, 1985).
Intensity
Cashmeran® demonstrates strong olfactory intensity with exceptional diffusive properties. Its typical use level in fine fragrance compositions ranges around 2%, significantly lower than traditional polycyclic musks like galaxolide (HHCB), which can be employed at concentrations up to 30%. This lower usage threshold reflects both the material’s potency and its role as a signature character contributor rather than a volume builder (Wikipedia, 2025).
The material exhibits substantivity of approximately 48 hours, indicating excellent tenacity on skin and textiles. With a Log P value of 4.20 and vapor pressure of 0.009316 mm Hg at 23°C, Cashmeran® displays ideal physical properties for sustained olfactory presence without excessive volatility (IFF, 2025).
Tenacity
The ingredient demonstrates exceptional longevity in formulations, classified as having long-lasting persistence. This extended tenacity makes Cashmeran® particularly valuable for creating depth and staying power in fragrance compositions. The material’s ketone functional group and polycyclic structure contribute to its ability to maintain olfactory presence throughout the fragrance development curve.
Volatility
Cashmeran® functions primarily as a middle to base note material with low volatility characteristics. Its boiling point ranges from 256°C to 286°C (depending on measurement conditions), though some decomposition may occur at temperatures above 220°C. The relatively low vapor pressure ensures gradual, sustained release of the odorant, making it particularly effective for heart and base note construction in perfumery (Wikipedia, 2025).
Fixative Properties
While not classified as a traditional fixative, Cashmeran® contributes significantly to fragrance structure through its ability to link and harmonize different olfactory elements. It serves as an effective bridge material, connecting musky softness with woody structure and helping to unify complex accords. The material excels in developing woody-amber complexes across all product categories and creates volume and warmth in both feminine and masculine fragrance types (IFF, 2025).
Applications in Fine Fragrance
Cashmeran® plays a versatile role in modern perfumery, employed across multiple fragrance families including orientals, woody musks, and contemporary florals. The material excels in creating spicy carnation florals, amber-musk notes, and woody accords. It provides essential character in developing woody-amber complexes and proves particularly useful in constructing the trendy oud and agarwood accords that define contemporary luxury perfumery (Kraft et al., 2020).
Notable fragrance compositions featuring prominent Cashmeran® usage include Maurice Roucel’s “Dans Tes Bras” (Frederic Malle, 2008) and Alessandro Gualtieri’s “Duro” (Nasomatto, 2007), both employing approximately 25% concentration. Other famous perfumes containing Cashmeran® include “Black Opium” (Yves Saint Laurent), “Blanche” (Byredo), “Bare Vanilla” (Victoria’s Secret), and “Good Girl” (Carolina Herrera).
The material demonstrates excellent blending characteristics with various perfumery materials. IFF documentation notes particularly successful combinations with Timbersilk®, citronellol, isoeugenol, and delta damascone. Arcadi Boix Camps observed interesting synergies with green grass notes, cis-3-hexenol derivatives, and Triplal, as well as with subtle fruity chemicals including ethyl levulinate, allyl caproate, methyl nicotinate, myrrh resinoid, and maté absolute.
Performance in Formula
In formulation, Cashmeran® exhibits stable behavior across various product matrices. The material maintains its olfactory profile across pH ranges typical of personal care and fine fragrance applications. Its compatibility extends to both alcohol-based and oil-based systems, making it suitable for diverse product formats from eau de parfum to functional fragrance applications.
The ingredient demonstrates good stability under normal storage conditions, though attention should be paid to preventing oxidation and thermal decomposition. The material’s relatively low volatility ensures consistent performance throughout product shelf life, contributing sustained woody-musky character without significant top-note loss during aging.
Industrial & Technical Uses
Beyond fine fragrance, Cashmeran® finds application in functional perfumery for personal care products, laundry care, and home fragrance. Its diffusive character and long-lasting properties make it particularly valuable for products where sustained olfactory presence is desired. The material’s stability profile supports its use in various consumer product categories including those exposed to elevated temperatures or challenging chemical environments.
Cashmeran® synthesis proceeds through oxidation of the corresponding pentamethyltetrahydroindane using oxygen in the presence of cobalt naphthenate catalyst. This industrial process allows for economical large-scale production while maintaining consistent quality standards for commercial supply.
Regulatory & Safety Overview
IFRA Status
Cashmeran® (DPMI) is subject to concentration restrictions under IFRA Amendment 49 (published 2020) and remains restricted in Amendment 51 (notified June 30, 2023). The restrictions are based on dermal sensitization and systemic toxicity considerations. Maximum acceptable concentrations vary by product category, ranging from 0.0063% for lipstick products (Category 1) to 9.4% for non-skin contact products (Category 12).
Implementation dates for existing creations extend to February 10, 2022 for Amendment 49 standards. The complete standard documentation is available at: https://ifrafragrance.org/standards/IFRA_STD_028.pdf
Maximum permitted concentrations by selected categories:
Category 1 (Lip products): 0.0063%
Category 2 (Deodorants/antiperspirants): 0.26%
Category 4 (Hydroalcoholic fine fragrance): 3.8%
Category 5A (Body lotions, face creams): 0.31%
Category 8 (Intimate wipes): 0.0084%
Category 11A (Diapers, toilet paper): 0.0084%
EU Cosmetics Regulation
Cashmeran® is classified with hazard statements H315 (skin irritation), H319 (eye irritation), and H317 (sensitization) under EU CLP regulation. Environmental classification includes H411 (harmful to aquatic life with long-lasting effects). The material is not classified as a CMR (carcinogenic, mutagenic, or reprotoxic) substance and is not considered toxic under current EU frameworks (Wikipedia, 2025).
Safety Profile
Toxicological assessment by the Research Institute for Fragrance Materials (RIFM) and the Expert Panel for Fragrance Safety establishes Cashmeran® as safe for use within specified concentration limits. The material exhibits slight skin irritation and eye irritation properties, with weak sensitization potential (EC3 of 33%).
Cashmeran® demonstrates a bioconcentration factor (BCF) of 156 and octanol-water partition coefficient (Log Kow) of 4.2, indicating it does not meet criteria for classification as very persistent, very bioaccumulative (vPvB) or persistent bioaccumulative toxic (PBT) substances. Short-term aquatic toxicity exceeds 1 mg/kg for all test species (Daphnia, algae, fish), placing it an order of magnitude more favorable than compounds typically classified as polycyclic musks (Wikipedia, 2025).
Additional Safety Information
The material is non-biodegradable and classified as vegan-suitable. Various environmental monitoring studies have been conducted in multiple compartments and human populations. The complete RIFM safety assessment is available through the RIFM Fragrance Material Safety Assessment Center at http://fragrancematerialsafetyresource.elsevier.com/.
References
Camps, A. B. (1985). Olfactory characterization of fragrance materials. Perfumer & Flavorist, 10(3), 35-42.
Hall, J. B. (1969). Pentamethylindane derivatives (U.S. Patent No. 3,773,836). United States Patent and Trademark Office.
International Flavors & Fragrances. (2025). Cashmeran® technical data sheet. Retrieved from https://www.iff.com/scent/ingredients-compendium/cashmeran/
International Fragrance Association. (2020). IFRA Standard: 6,7-Dihydro-1,1,2,3,3-pentamethyl-4(5H)-indanone (DPMI) (Amendment 49). Retrieved from https://ifrafragrance.org/standards/IFRA_STD_028.pdf
International Fragrance Association. (2023). Index of IFRA Standards – 51st Amendment. Retrieved from https://ifrafragrance.org/docs/default-source/51st-amendment/ifra-51st-amendment—index-of-ifra-standards.pdf
Kraft, P., Bajgrowicz, J. A., Denis, C., & Frater, G. (1998). Fragrance chemistry. Tetrahedron, 54(25), 7633-7703.
Kraft, P., Armanino, N., Charpentier, J., & Flachsmann, F. (2020). What’s hot, what’s not: The trends of the past 20 years in the chemistry of odorants. Angewandte Chemie International Edition, 59(52), 23070-23095.
Ohloff, G., Pickenhagen, W., & Kraft, P. (2012). Scent and chemistry: The molecular world of odors. Verlag Helvetica Chimica Acta and Wiley-VCH.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Wiley-VCH. pp. 85-86.
Wikipedia. (2025). Cashmeran. Retrieved from https://en.wikipedia.org/wiki/Cashmeran