Aldehydes not aldehydes
After delving into the discourse of aldehydes in this article, we have been asked several times why aldehydes 14, 16, and 18 are not included in the list. The answer is quite simple. These products are not aldehydes, although historically we have maintained (wrongly) or otherwise for marketing reasons, the word aldehyde.
Differences between aldehydes and lactones in perfumery
Introduction
As we know, perfumery is an art form. It involves mixing different ingredients to create new scents that appeal to our sense of smell. There are many different types of molecules that perfumers use, but two groups are most common: aldehydes and lactones. Both groups have their uses in making perfume and both have their own unique characteristics as well. In this article, I will talk about the similarities and differences between these two groups in order to better understand them and make good scent choices when creating perfume formulas.
We will be diving into the differences between these two groups of ingredients that are often wrongly confused.
You’re probably familiar with the aromatic aldehydes and lactones that are commonly used in perfumery and food, but you might not know why these two groups of ingredients are so different. We will discuss what these two groups of ingredients are and how they differ from one another. We’ll also talk about the uses of aromatic aldehydes and lactones in perfumery and how they can be used to create unique scents that make your perfume stand out.
We’re going to dive into the differences between these two groups of ingredients that are often wrongly confused. This should be eye-opening for anyone who thinks they know everything there is to know about scent chemistry!
Before we start, let’s quickly recap what these molecules are and how they work.
Aldehydes are organic compounds with a carbonyl group at the end of the carbon chain. They are oxygenated compounds and a class of organic compounds that can be derived from the oxidation of primary alcohols. A typical example is formaldehyde, which smells like formaldehyde naturally found in living things (don't worry, it's not going to give you cancer). Aldehydes can also be formed during polymerization reactions or by catalytic hydrogenation of acids or other terminal oxidizable groups on aromatic rings. In perfumery, aldehydes have long been popular as fragrances which often have green notes characteristic of freshly cut grass; however, lately, there has been an increased interest in lactones as well.
Aldehydes
aldehyde, any of a class of organic compounds in which a carbon atom shares a double bond with an oxygen atom, a single bond with a hydrogen atom, and a single bond with another atom or group of atoms (designated R in general chemical formulas and structure diagrams). The double bond between carbon and oxygen is characteristic of all aldehydes and is known as the carbonyl group.
In perfumery, they are used to give floral notes a special kick, green notes, and as boosters to fragrances.
Lactones
In perfumery, lactones are cyclic esters that give fragrances a fruity and milky note. They are also called lactides because they can be found in dairy products like butter and cream.
Lactones, any of a class of cyclic organic esters, are usually formed by the reaction of a carboxylic acid group with a hydroxyl group or halogen atom present in the same molecule. Commercially important lactones include diketene and β-propanolactone used in the synthesis of acetoacetic acid derivatives and β-substituted propanoic (propionic) acids, respectively; the perfume ingredients pentadecanolide and ambrettolide. The γ- and δ-lactones, containing five- and six-membered rings, respectively, are the most common. They are formed by loss of water from the corresponding hydroxy acids, a process that often occurs spontaneously even in an aqueous solution.
What does this mean? An aldehyde group occurs at the terminal end of a carbon chain while a lactone group occurs at an internal carbon atom within a chain. In other words, a lactone is an internal ring within a chain while an aldehyde is always at the end of a chain.
Aldehydes and lactones are both similar in that they are used frequently as top notes. They can be used interchangeably and in combination with other fragrant compounds to create unique scents. But what does it mean?
Lactones have very different properties depending on their location within the molecule: some are more fruity and sweet, while others have floral or spicy qualities—a result of their placement along the length of the molecule’s backbone. Aldehydes can also have these qualities; however, their characteristic qualities tend to remain intact regardless of where they are positioned in relation to another part of its structure (that is why we consider them “primary volatile components”).
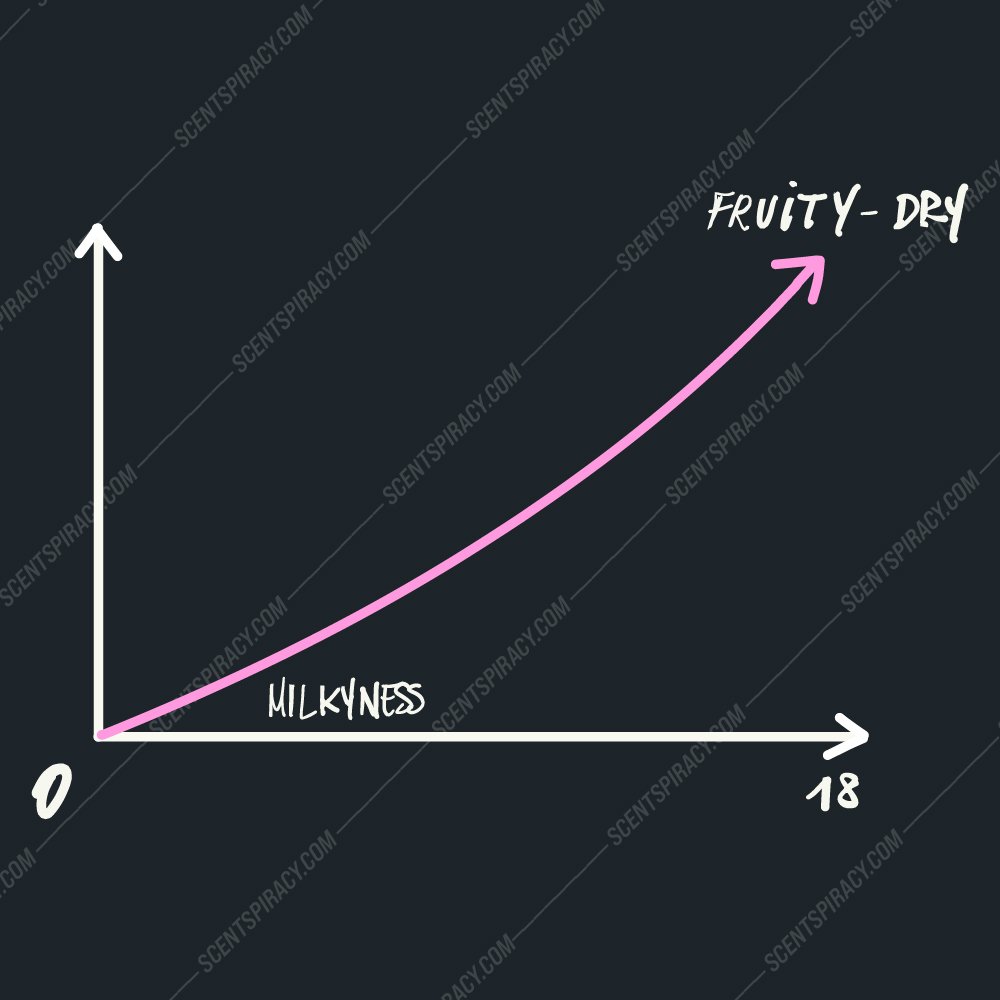
Lactones smell fruity and milky depending on the length of the chain
Lactones are characterized by the presence of a carbon-oxygen double bond in the ring and a ketone group on the side chain. In perfumery, lactones have a very specific smell: they’re used for their fruity and milky notes. In food products, they’re known for giving yogurt its creamy consistency. In cosmetics, they help make suntan lotion feel luxurious—in fact, any product that has “lactic acid” in its ingredients likely contains some lactone!
Lactones can come in different lengths and structures depending on where it is on the molecule (the name comes from how long it is). This means that even though all lactones have similar smells, there are slight differences between one type of lactone and another (and even within each type).
Lactones chain length graph ( scentspiracy idea )
Conclusion
With the right skills and knowledge, you can make the most of aldehydes or lactones in perfumery.
In conclusion, we can say that lactones and aldehydes are very different molecules. They belong to different chemical families and they have different properties. There are many more differences between the two that we did not discuss here but this article should give you a good idea about their main differences and similarities so you will be able to distinguish them easily in your perfumery work!
Sources and Information:
Fulvio Ciccolo, Scentspiracy (2022)
Brown, W. H. and March, . Jerry (2022, October 14). aldehyde. Encyclopedia Britannica. https://www.britannica.com/science/aldehyde
Brown, W. H. (2010, August 3). lactone. Encyclopedia Britannica. https://www.britannica.com/science/lactone