Synthetic Ingredient Overview
🏭 Manufacturer: Industrial synthesis from α-pinene
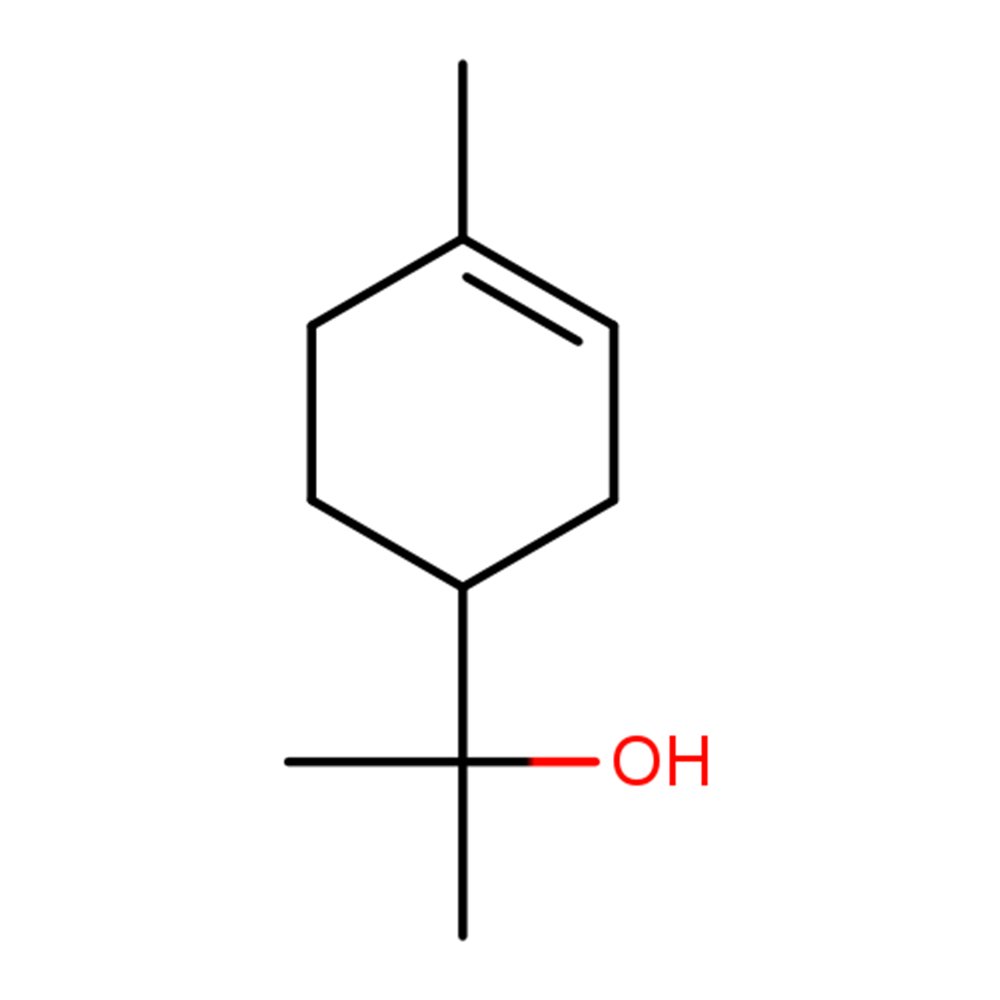
🔎 IUPAC Name: 2-(4-Methyl-1-cyclohex-3-enyl)propan-2-ol
🧪 Synonyms: α-Terpineol, β-Terpineol, γ-Terpineol
🧬 Chemical Formula: C₁₀H₁₈O
📂 CAS N°: 98-55-5 (α), 138-87-4 (β), 586-81-2 (γ)
📘 FEMA: 3049
⚖️ Molecular Weight: 154.25 g/mol
📝 Odor Type: Fresh (Terpenic)
📈 Odor Strength: Medium – ~20 hrs on strip
👃🏼 Odor Profile: Clean, fresh, lilac, terpenic, with dirty undertone and citrus-soapy character
👅 Flavor Profile: Citrus, lemon-lime, woody, soapy
⚗️ Uses: Terpenic modifier, floral booster, pine/fougère core, flavoring in citrus, coffee, tomato, and apple accords
🧴 Appearance: Colorless to pale liquid (α-isomer)
What is Terpineol?
Terpineol refers to a family of cyclic monoterpene alcohols with isomeric forms (α, β, γ), typically derived from the hydration of α-pinene, a major constituent of turpentine. These alcohols occur naturally in pine, petitgrain, and cajuput oil, but most commercial material is semi-synthetic.
The most widely used isomer, α-Terpineol, features a lilac-like floral scent, useful in both fragrance and flavor applications. It serves as a clean floral top note with body, bridging citrus openings to green or floral hearts.
Olfactory Profile & Perfumery Applications
Position in Fragrance Structure: Top-to-heart
👃🏼 Detailed Breakdown:
Top: Terpenic citrus-clean (lemon-lime nuance)
Heart: Lilac, floral-green, faintly soapy
Base: Light balsamic dryout with residual cleanness
⚗️ Functional Use:
Floral systems: Core modifier in lilac, muguet, neroli, peony-type florals
Fougères and pine accords: Supports coniferous sharpness in masculine structures
Household and detergent perfumery: Signature “clean” note
Low-cost soap base construction: Compatible with aldehydes, coumarins, ionones
🔬 Effective in combination with:
Linalool, citronellol (adds floralcy)
Lemon oil, d-limonene (reinforces citrus)
Isobornyl acetate, pine oil (coniferous elevation)
Coumarin, oakmoss, methylionones (fougère bases)
Industrial & Technical Uses
Fine Fragrance: Occasionally used in retro-style florals or green florals
Functional Fragrance: Widely applied in soaps, detergents, air care
Flavor Use (FEMA 3049):
Fruit (apple, lemon, peach)
Beverage and spice modifiers
Used in flavor types like coffee, tomato, citrus
Pharmaceutical/Cosmetic: Exhibits antimicrobial, anti-inflammatory activity; acts as a penetration enhancer
Regulatory & Safety Overview
IFRA: Not restricted under current IFRA 51st Amendment
EU Allergens: Not listed among 26 declarable allergens (verify trace levels)
FEMA GRAS: FEMA 3049 – approved for flavor use
ECHA (REACH): Registered and not classified as hazardous
Toxicology:
Low irritancy under normal usage
Biodegradable
No evidence of phototoxicity or sensitization at normal perfumery dosage
✅ Considered safe in fragrance, cosmetic, and food use when compliant with IFRA/FEMA guidelines.
Sources
Adams, R. P. – Identification of Essential Oil Components by GC/MS
FEMA GRAS Database – Terpineol (3049)
ECHA Substance Information – 98-55-5
Poucher's Perfumes, Cosmetics and Soaps – 10th ed.
Internal evaluation – Scentspiracy Team, Fulvio Ciccolo 2023