Helvetolide® – Technical Ingredient Overview
🏭 Manufacturer — Firmenich (DSM-Firmenich)
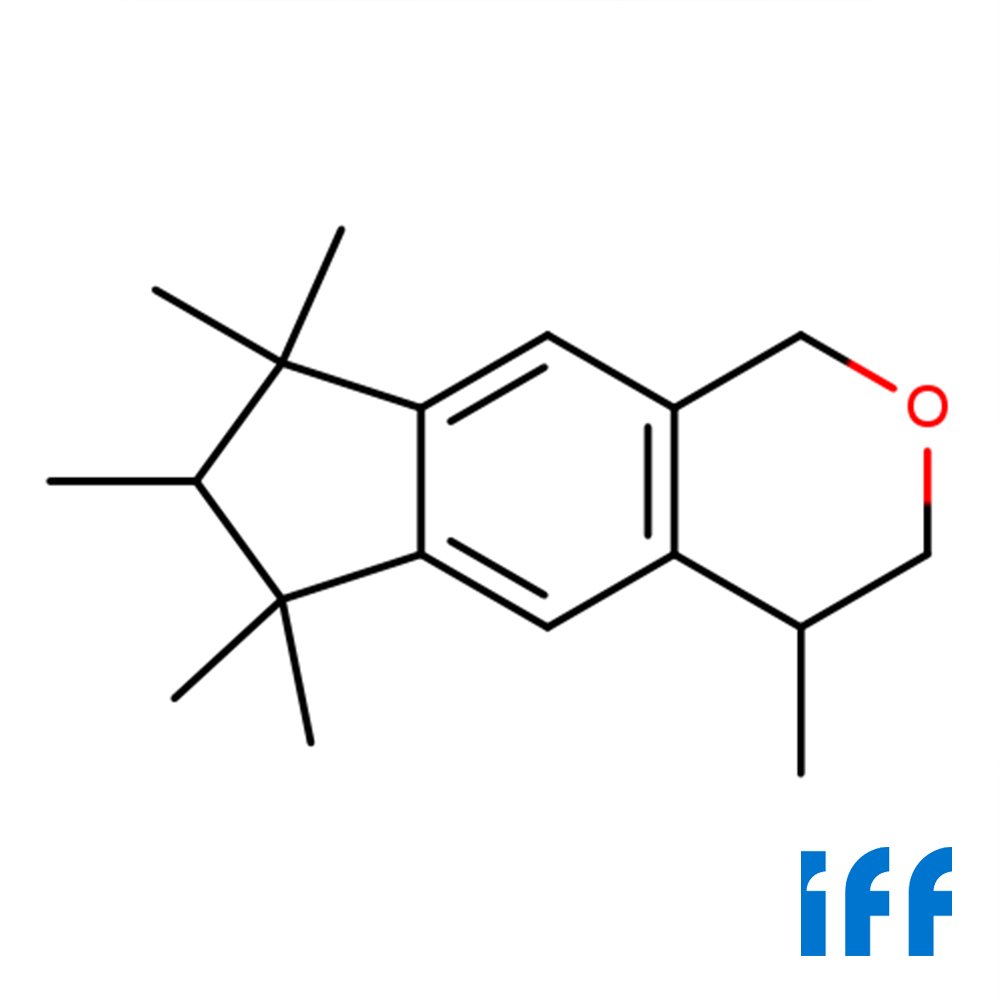
🔎 Chemical Name — 2-[1-(3,3-Dimethylcyclohexyl)ethoxy]-2-methylpropyl propionate
🧪 Synonyms — 1-Propanol, 2-[1-(3,3-dimethylcyclohexyl)ethoxy]-2-methyl-, 1-propanoate; 1-Propanol, 2-[1-(3,3-dimethylcyclohexyl)ethoxy]-2-methyl-, propanoate; Musk propanoate; Dimethylcyclohexylethoxy isobutylpropanoate; Alicyclic musk; Linear musk
📂 CAS Number — 141773-73-1
📘 FEMA Number — Not applicable (fragrance use only)
📊 EINECS Number — 415-490-5
⚖️ Molecular Weight — 284.43 g/mol
🧬 Molecular Formula — C₁₇H₃₂O₃
📝 Odor Type — Musky, fruity, ambrette-like
📈 Odor Strength — High (radiant and diffusive)
👃🏼 Odor Profile — Sophisticated modern musk with distinctive fruity pear facets and smooth ambrette seed undertones; clean, white, milky character with unexpected volume and richness; warm and elegant
⚗️ Uses — Fine fragrance formulations, functional fragrances, cosmetics, personal care products (shampoos, body washes), household products
🧴 Appearance — Colorless liquid
🌡️ Boiling Point — 139°C
🔥 Flash Point — >100°C
💧 Density — 0.94 g/cm³
🔬 Vapor Pressure (20°C) — 0.02 Pa
💦 Solubility — Soluble in alcohol; not soluble in water
⏱️ Tenacity on Blotter — 2 weeks (>400 hours)
What is Helvetolide®?
Helvetolide® (CAS 141773-73-1) is a synthetic alicyclic musk developed by Firmenich, representing a significant advancement in modern perfumery chemistry. This fourth-generation musk odorant belongs to the alicyclic (or linear) musk family, which emerged as an innovative alternative to earlier nitro musks, polycyclic musks, and macrocyclic musks (Kraft, P., 2004).
Chemically, Helvetolide is characterized by its molecular formula C₁₇H₃₂O₃ and systematic IUPAC name: 2-[1-(3,3-dimethylcyclohexyl)ethoxy]-2-methylpropyl propionate. The molecule features a distinctive structural architecture combining a 3,3-dimethylcyclohexyl moiety connected via an ethoxy linkage to a branched propanol core, which is further esterified with propanoic acid. This unique configuration contributes to its exceptional olfactory properties and performance in perfume formulations.
The commercial product exists as a mixture of two stereoisomers due to the presence of two asymmetric carbon atoms in the molecule. The dextrorotatory isomer Helvetolide® (+) exhibits less floral but more musky characteristics compared to the levorotatory isomer Helvetolide® (-). The (+) enantiomer is often preferred in fine fragrance compositions and is sometimes used in isolation for specific applications.
Historical Background
Helvetolide® was discovered in 1990 by chemists Giersch and Schulte-Este at Firmenich, marking a pivotal moment in fragrance chemistry innovation. The compound was officially launched in 1991 and represented the first alicyclic musk to be produced at commercial scale, though compounds of this structural class had been identified prior to 1980.
The trademark 'Helvetolide®' was published and protected by Firmenich SA on April 30, 1995 (brand N°636238), with additional trademark protection established on December 12, 2001 (brand N°772121). The name Helvetolide honors the Helvetic Confederation (Switzerland), reflecting Firmenich's Swiss heritage.
Helvetolide® is recognized as the first alicyclic musk used commercially in perfumery, opening an entirely new category of musk molecules. Following its introduction, related alicyclic musks including Romandolide®, Nebulone®, Edenolide®, and Sylkolide® were subsequently developed, establishing alicyclic musks as the fourth generation of synthetic musks in the industry (David, O.R.P., 2020).
The molecule's discovery emerged during a period when the fragrance industry was actively seeking biodegradable, safe, and high-performance alternatives to earlier musk generations that faced environmental and regulatory challenges.
Olfactory Profile
Scent Family: Alicyclic musk with fruity-ambrette characteristics
Main Descriptors:
Excellent volatile pear-like facets with smooth ambrette seed undertones
Clean, white, milky musk character with modern sophistication
Richness and warmth reminiscent of natural ambrette seeds
Subtle yogurt-like acidic nuance in the fruity pear facet
Modern, sophisticated, and refined musky base
Intensity: Highly diffusive and radiant; even trace amounts can have enormous impact on perfume compositions
Tenacity: Exceptional longevity with 2-week persistence on blotter (>400 hours)
Volatility: Unique among musks for its top note presence while maintaining base note persistence; functions as a top-to-base modifier
Classification: Olfactively positioned between Ambrettolide (more floral) and Ethylene Brassylate/Musk T (more base-heavy)
Fixative Role: Acts as a fixative without suppressing other fragrance notes; adds presence to top notes while extending throughout the fragrance development
Applications in Fine Fragrance
Helvetolide® functions as a top-to-base modifier in perfumery, delivering tenacity and longevity without overwhelming floral or fruity compositions. Its distinctive ability to impact both the opening and the drydown makes it invaluable for creating modern, sophisticated fragrance structures.
Role in Compositions:
Enhances floral accords and smooths fruity notes without functional connotations
Excellent addition to gourmand fragrances due to its delicate pear nuance
Works synergistically with other musks when used in combination, particularly effective with Ambrettolide, Romandolide, and Exaltolide
Creates clean musk accords that retain sensuality and elegance
Notable Fragrance Accords:
Combined with 8% Habanolide creates the Emporio Armani White She accord
Used alongside Habanolide in Emporio Armani White For Her by perfumer Alberto Morillas (2001), helping establish the modern "white musk" category
Pairs effectively with floral, fruity, woody, amber, and ozonic notes
Performance in Formula
Helvetolide® performs particularly well in liquid fragrance applications including alcohol-based perfumes, shampoos, and body washes, as well as in emulsions. It performs exceptionally in both leave-on and rinse-off products without breakdown or olfactory flattening.
Blending Characteristics:
Increases power and diffusion when combined with other musks
Compatible with alcohol-based formulations and various functional bases
Stable in perfume compositions and various functional bases
Works particularly well with Habanolide, Exaltolide, Ambrettolide, Musk T, Applelide, Edenolide, and Romandolide
Typical Usage Levels: 0.5% - 5% in finished formulations
Chemical Stability: The ester functional group provides good stability under typical formulation conditions while allowing for controlled hydrolysis in environmental degradation pathways.
Industrial & Technical Uses
Beyond fine fragrance, Helvetolide® is widely used in perfumes and personal care products to enhance odor character and tenacity. Applications include:
Personal Care Products:
Body care formulations (shower gels, lotions, body washes)
Hair care products (shampoos, conditioners)
Fine soaps and cleansers
Household Products:
Air fresheners, scented candles, fabric softeners, and detergents
Laundry care products
Home fragrance applications
Chemical Research:
Employed as a reagent in organic synthesis for creating various ester derivatives
Can be used as a precursor to synthesize Romandolide®, achieved by replacing the dimethyl group of the ether function with a carbonyl function
Sustainability & Green Chemistry: DSM-Firmenich utilizes upcycled turpentine extracted from the paper industry (softwood sources for cardboard manufacturing). This turpentine undergoes fractionation to retrieve alpha and beta pinene, the primary starting materials for Helvetolide® synthesis. This approach aligns with green chemistry principles and reduces reliance on petroleum-based feedstocks.
Regulatory & Safety Overview
IFRA Status: Not restricted under IFRA Amendment 51. Permitted with standard usage limits that are category-specific. Contains no allergens.
Official IFRA documentation: https://ifrafragrance.org/safe-use/library
EU Cosmetics Regulation (EC) No 1223/2009: Compliant with EU 1223/2009 regulations for cosmetic use. EINECS Number: 415-490-5
Environmental Profile:
Studies have demonstrated that Helvetolide can be biodegraded by specific microbial strains in environmental settings, indicating suitability for green chemistry applications
Part of the biodegradable alicyclic musk family, addressing environmental concerns associated with earlier polycyclic musks
Stable in perfumes and various functional bases under normal storage and usage conditions
GHS Classification: Standard handling procedures apply for fragrance ingredients. Flash point >100°C indicates relatively low flammability hazard
Handling & Storage:
Store in a cool, dry, ventilated area, away from heat and direct sunlight
Use gloves and eye protection to avoid skin and eye irritation
Standard precautions for handling fragrance raw materials apply
Toxicology: Biodegradation studies suggest environmentally favorable breakdown pathways through microbial action. The compound undergoes hydrolysis of the ester group to release alcohol and acid components that can interact with biological pathways.
Sources
CAS Common Chemistry. (n.d.). Helvetolide. Retrieved from https://commonchemistry.cas.org/detail?cas_rn=141773-73-1
David, O.R.P. (2020). A chemical history of polycyclic musks. Chemistry – A European Journal, 26(34), 7537-7555. https://doi.org/10.1002/chem.202000577
DSM-Firmenich. (n.d.). Discover our story. Retrieved from https://www.firmenich.com/en-cn/company/discover-our-story
DSM-Firmenich. (n.d.). Helvetolide® technical information. Retrieved from https://www.firmenich.com/product/helvetolider-pe-947650-0
Kraft, P. (2004). Aroma chemicals IV: Musks. In D.J. Rowe (Ed.), Chemistry and Technology of Flavors and Fragrances. Blackwell Publishing Ltd., Oxford, UK.
Kraft, P., & Eh, M. (2004). New alicyclic musks: The fourth generation of musk odorants. Chemistry & Biodiversity, 1(12), 1975-1984. https://doi.org/10.1002/cbdv.200490151
UL Solutions. (2023, June 30). IFRA notifies 51st amendment to the IFRA standards. Retrieved from https://www.ul.com/news/ifra-notifies-51st-amendment-ifra-standards
Wikipedia contributors. (2025, June 24). Synthetic musk. In Wikipedia, The Free Encyclopedia. Retrieved from https://en.wikipedia.org/wiki/Synthetic_musk