Menthol (2216-51-5) Synthetic Ingredient Overview
🏭 Manufacturer — Multiple (Takasago, Symrise, others)
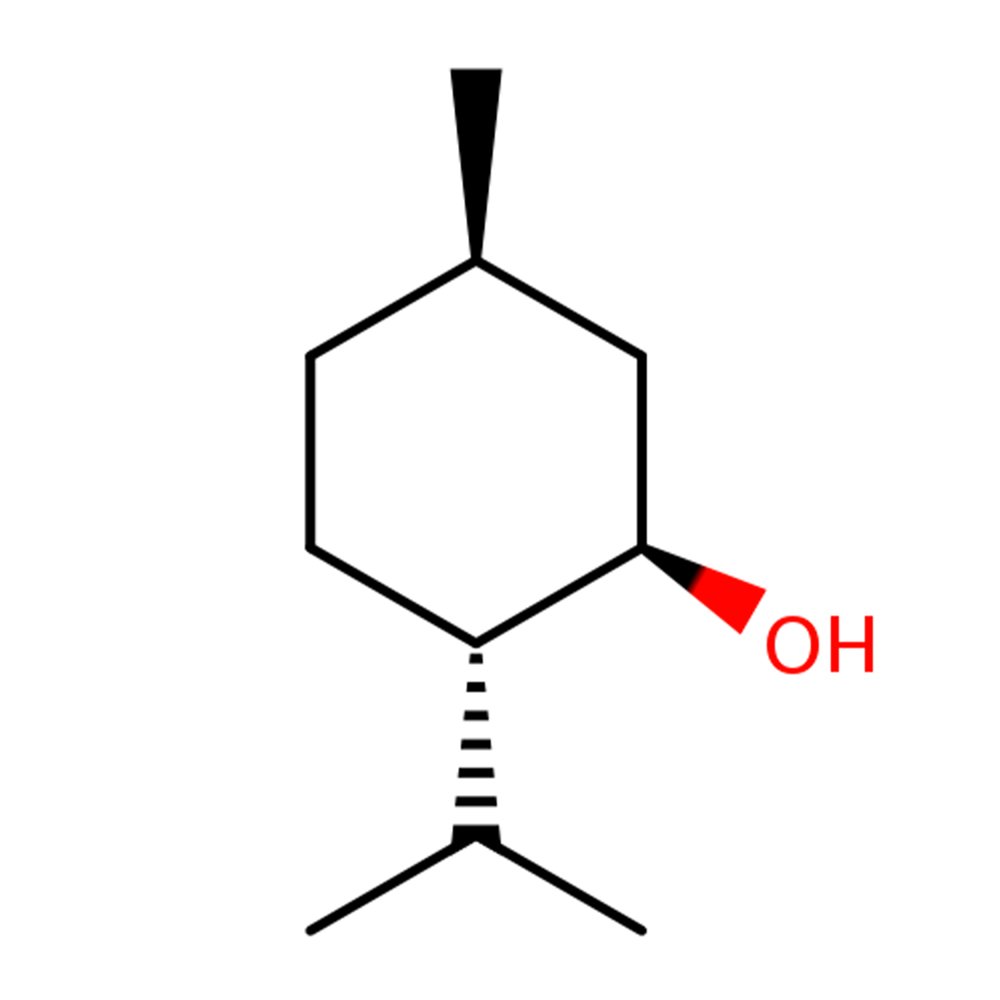
🔎 Chemical Name — 2-Isopropyl-5-methylcyclohexanol (IUPAC: (1R,2S,5R)-(−)-Menthol for natural form)
🧪 Synonyms — Menthol, (−)-Menthol, l-Menthol, dl-Menthol (racemic), mint camphor, peppermint camphor
🧬 Chemical Formula — C₁₀H₂₀O
📂 CAS Number — 2216-51-5 (racemic), 89-78-1 (l-Menthol)
📘 FEMA Number — 2665
⚖️ Molecular Weight — 156.27 g/mol
🌡️ Melting Point — 42-43°C (l-menthol), 38°C (dl-menthol)
🔥 Boiling Point — 216.5°C at 101.3 kPa
🔬 Refractive Index — n²⁰D 1.4600 (l-menthol)
📝 Odor Type — Fresh, minty, cooling
📈 Odor Strength — High, diffusive
👃🏼 Odor Profile — Peppermint-like, sweet, clean, cooling, slightly herbaceous
⚗️ Uses — Cooling modifier, top note booster, freshness enhancer, flavoring agent, TRPM8 receptor activator, functional ingredient
🧴 Appearance — White crystalline solid or clear needles; melts ~36–43°C
What is Menthol?
Menthol is a monocyclic monoterpene alcohol that occurs naturally in mint plants or is produced synthetically through various chemical routes. It exists as eight stereoisomers due to three asymmetric carbon atoms, with (−)-menthol (also called l-menthol) being the most commercially important form because it produces the strongest physiological cooling effect (Surburg & Panten, 2006).
Structurally, menthol adopts a stable chair conformation that allows its hydroxyl, methyl, and isopropyl groups to occupy equatorial positions, maximizing both physical and sensory stability. This molecular arrangement is key to menthol's unique dual functionality—it simultaneously activates olfactory receptors (producing the mint smell) and cold-sensing TRPM8 ion channels (creating the cooling sensation) at different concentration thresholds (Rowe, 2005).
Natural menthol is primarily isolated from Mentha arvensis (cornmint or Japanese mint), which can contain 70-85% menthol, while Mentha piperita (peppermint) typically contains 40-50% menthol. Synthetic menthol, produced through hydrogenation of thymol, citronellal, or pulegone, now accounts for a significant portion of global supply, offering superior stereoisomeric control and cost efficiency.
Historical Background
Menthol was first isolated in 1771 by Hieronymus David Gaubius, a German botanist working in the Netherlands, marking one of the earliest isolations of a pure compound from essential oils (Kamatou et al., 2013). However, mint plants containing menthol had been cultivated and used medicinally in Japan for centuries before this isolation—Japanese traditional medicine extensively employed mint preparations for digestive ailments and cooling effects.
The compound was formally named "menthol" in 1861 by F. L. Alphons Oppenheim (1833-1877), deriving from the Latin mentha (mint). Early characterization work was conducted by scientists including Oppenheim, Beckett, Moriya, and Atkinson throughout the 19th century, establishing menthol's chemical structure and stereoisomeric complexity (Read, 1930).
By the late 19th century, Japan had become the world's dominant producer of natural menthol through large-scale cultivation of Mentha arvensis. In 1938, the Kitami region of Hokkaido, Japan, produced an astonishing 70% of the world's mint oil supply. This Japanese dominance continued until World War II, when supply disruptions forced Western companies like Vick Chemical Company to develop extraction methods from American peppermint oil in 1942.
The first major synthetic route was developed in the early 20th century, with significant advances coming in the 1980s when Takasago International Corporation, under the leadership of Nobel Prize winner Ryōji Noyori, developed an asymmetric synthesis process capable of producing (−)-menthol at 94% enantiomeric excess on an industrial scale of 3,000+ tonnes annually (Noyori et al., 2001). Today, global menthol production exceeds 30,000 tonnes per year, with both natural extraction (primarily from India and China) and synthetic production playing major roles.
Olfactory Profile
Scent Family
Primary: Fresh, Minty
Secondary: Herbaceous, Camphoraceous
Main Descriptors
Primary: Fresh, clean mint, cooling, penetrating
Secondary: Sweet, slightly herbaceous, camphoraceous undertone, Peppermint-like clarity without harsh menthone bitterness
Menthol delivers an immediately recognizable fresh mint aroma that most people associate with oral care products, chewing gum, and medicinal preparations. Unlike some mint materials, pure menthol has a clean, sweet character without the bitter or harsh notes that can come from high menthone content.
Intensity
High. Menthol is extremely diffusive and detectable at very low concentrations—the olfactory threshold is sub-ppm (parts per million), meaning people can smell menthol at concentrations below 1 mg/kg.
Tenacity
Low to medium. As a relatively volatile monoterpene alcohol (boiling point 216.5°C), menthol evaporates fairly quickly and functions primarily as a top note material, lasting 1-3 hours on a blotter.
Volatility
Top note material with medium-high volatility for its molecular weight. The cooling sensation threshold (~0.3 g for oral perception) is higher than the olfactory threshold, meaning menthol can be smelled before the cooling effect is felt (Eccles, 2005).
Cooling Mechanism
Menthol's most distinctive property is its physiological cooling effect, mediated by TRPM8 (transient receptor potential melastatin 8) cold-sensing receptors. This creates a sensation of coolness without actual temperature change—a unique property that l-menthol exhibits 45 times more strongly than d-menthol (Rowe, 2005).
Applications in Fine Fragrance
Menthol serves multiple roles in perfumery, from functional cooling effects to olfactory freshness:
Fougères and Aromatic Compositions: Enhances lavender, geranium, and herbal notes with minty freshness
Fresh Florals: Lifts and brightens heavy floral bouquets like rose and jasmine
Functional Perfumery: Essential in shaving products, aftershaves, cooling gels, and sport fragrances
Mint Accords: Core ingredient in spearmint, peppermint, and menthol systems
Cooling Contrast: Adds refreshing counterpoint to warm spices and camphoraceous materials
Typical dosage in perfume concentrates: 0.5-2.5% for freshening and lifting effects. Menthol pairs well with eucalyptol, lavender, rosemary, citrus oils, and other aromatic materials.
Performance in Formula
Dosage and Blending
Concentration Range: 0.5-2.5% in fine fragrance concentrates; up to 5% in functional applications
Threshold Effects: Cooling sensation begins at ~0.1-0.5% on skin; olfactory impact detectable at much lower levels
Synergies: Blends beautifully with camphor, eucalyptol, pine, citrus, and aromatic herbs
Formulation Behavior
Menthol is solid at room temperature but melts easily at 36-43°C, making it simple to incorporate into alcoholic perfume bases by gentle heating or pre-dissolving in warm ethanol. It shows excellent stability in most cosmetic bases, though it can evaporate over time in open containers due to its volatility.
Stereochemistry in Commercial Products
Commercial menthol exists as either enantiomerically pure (−)-menthol or racemic mixtures depending on production method and intended use. Natural menthol from Mentha arvensis is predominantly (−)-menthol with small amounts of (+)-neomenthol. Synthetic routes can yield either pure enantiomers or racemic mixtures, with racemic menthol having slightly different physical properties (melting point 38°C vs. 42-43°C for pure l-menthol) but similar odor profiles (Surburg & Panten, 2006).
Chemical Reactions
Menthol readily undergoes esterification with carboxylic acids to form menthyl esters (e.g., menthyl acetate, menthyl benzoate), which are themselves valuable fragrance materials. Oxidation yields menthone, while dehydration produces p-menthenes. These reactions are exploited industrially to convert between different mint constituents.
Industrial & Technical Uses
Perfumery and Cosmetics
Personal care: Shampoos, shower gels, body lotions, cooling creams
Oral care: Toothpastes, mouthwashes, breath fresheners (major application)
Shaving: Aftershaves, pre-shave preparations, cooling gels
Functional: Sport fragrances, muscle rubs, topical analgesics
Flavor Industry
Chewing gum: 1000-1200 ppm (highest permitted concentration)
Confectionery: Hard candies, mints, chocolates (35-400 ppm)
Beverages: Mint teas, flavored waters, cocktails
Tobacco: Menthol cigarettes (~25% of global cigarette market)
Pharmaceutical Applications
Topical analgesics and muscle rubs (cooling and pain relief)
Cough and cold preparations (nasal decongestant effects)
Oral medications (flavor masking agent)
Transdermal penetration enhancer
Biological Properties
Beyond its sensory effects, menthol exhibits diverse pharmacological activities:
TRPM8 agonist: Activates cold-sensing receptors for cooling sensation
κ-opioid receptor agonist: Contributes to mild analgesic effects
GABA_A positive allosteric modulator: Mild sedative/anesthetic properties
Voltage-gated sodium channel blocker: Reduces neuronal excitability
Antimicrobial: Active against Streptococcus and Lactobacillus species (Kamatou et al., 2013)
Regulatory & Safety Overview
FEMA GRAS Status: 2665 – Generally Recognized as Safe for food use
IFRA Status: Permitted for use in fragrances with product-specific limitations. Not restricted under IFRA Standards 51st Amendment (2024) for typical fragrance applications. Standard safety assessments apply.
EU Cosmetics Regulation (EC) No 1223/2009: Approved for use in cosmetic products. Not listed among the 26 declarable fragrance allergens.
REACH Registration: Registered with ECHA. Not classified as hazardous under normal handling and use conditions.
Toxicology:
LD₅₀ (oral, rat): 3,300 mg/kg
LD₅₀ (oral, mouse): 3,400 mg/kg
Non-carcinogenic, non-mutagenic in standard testing
Potential irritant at very high concentrations (>5%)
Can cause mucous membrane discomfort at excessive doses
Generally well-tolerated; survival after doses of 8-9 g has been reported in humans
Safety Precautions:
Avoid use on face/chest of infants and young children (menthol inhalation risk)
May cause heartburn when taken orally in large amounts
Store below melting point in sealed containers away from heat
Flammable in vapor form; follow standard perfumery safety protocols
✅ Menthol is considered safe for use in fragrances, flavors, and cosmetics when used according to good manufacturing practices and within recommended concentration ranges.
References
Eccles, R. (2005). Menthol and related cooling compounds. Journal of Pharmacy and Pharmacology, 46(8), 618-630.
European Chemicals Agency (ECHA). (n.d.). Substance information: Menthol, CAS 89-78-1. Retrieved from https://echa.europa.eu/
International Fragrance Association (IFRA). (2024). IFRA Standards – 51st Amendment. Retrieved from https://ifrafragrance.org/safe-use/standards-documentation
Kamatou, G. P. P., Vermaak, I., Viljoen, A. M., & Lawrence, B. M. (2013). Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry, 96, 15-25. https://doi.org/10.1016/j.phytochem.2013.08.005
Noyori, R., Ohkuma, T., Kitamura, M., Takaya, H., Sayo, N., Kumobayashi, H., & Akutagawa, S. (2001). Asymmetric hydrogenation of β-keto esters. A practical, purely chemical access to β-hydroxy esters in high enantiomeric purity. Journal of the American Chemical Society, 110, 629-631.
Read, B. E. (1930). A brief history of menthol. Journal of Chemical Education, 7(12), 2755-2759.
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Blackwell Publishing.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Wiley-VCH.