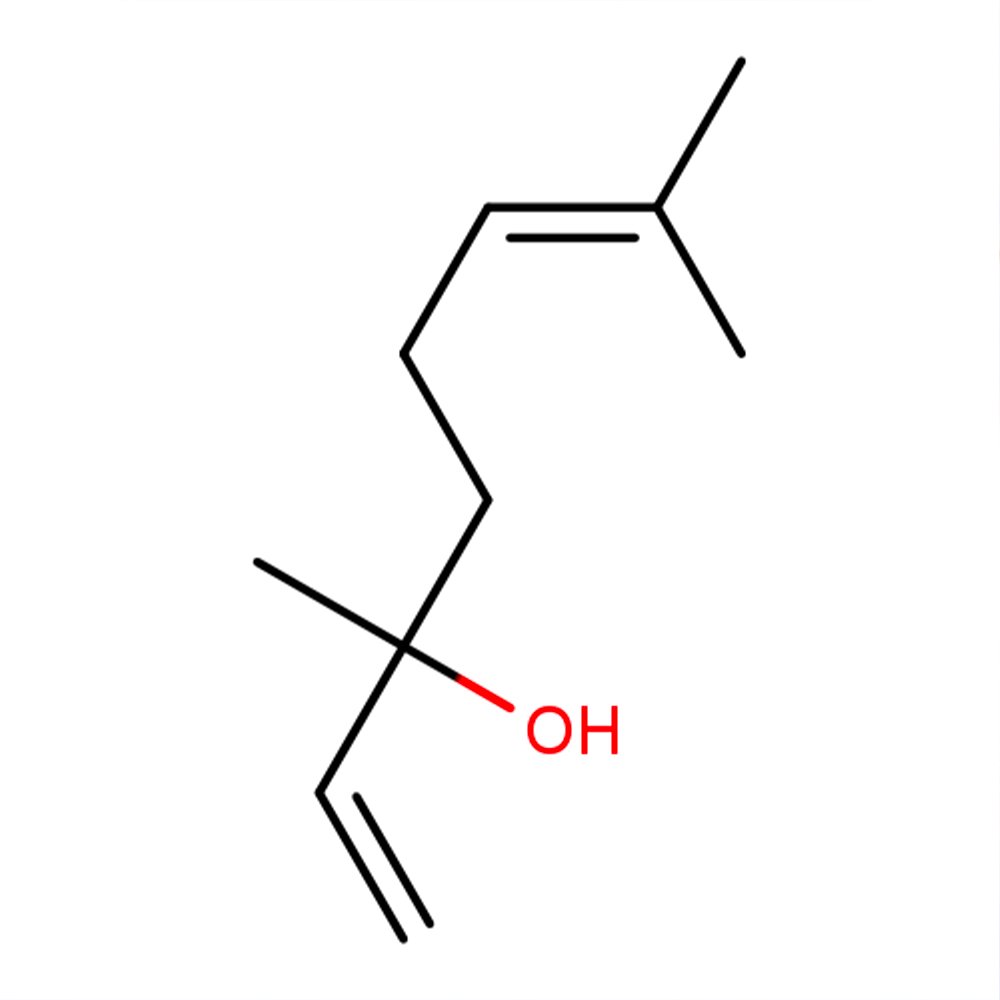
 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Linalool
Premium Synthetic Ingredient for Perfumery
Linalool is a widely used terpene alcohol with a floral-woody odor and a faint citrus undertone. Naturally occurring but industrially produced via synthetic or semi-synthetic methods, it serves primarily as a top-note enhancer, a floral modifier, and a technical blender. Its versatile scent harmonizes with citrus, fruit, spice, and floral materials. Though poor in tenacity, its diffusive freshness brings lift and naturality to a broad spectrum of perfumery styles.
Premium Synthetic Ingredient for Perfumery
Linalool is a widely used terpene alcohol with a floral-woody odor and a faint citrus undertone. Naturally occurring but industrially produced via synthetic or semi-synthetic methods, it serves primarily as a top-note enhancer, a floral modifier, and a technical blender. Its versatile scent harmonizes with citrus, fruit, spice, and floral materials. Though poor in tenacity, its diffusive freshness brings lift and naturality to a broad spectrum of perfumery styles.
Premium Synthetic Ingredient for Perfumery
Linalool is a widely used terpene alcohol with a floral-woody odor and a faint citrus undertone. Naturally occurring but industrially produced via synthetic or semi-synthetic methods, it serves primarily as a top-note enhancer, a floral modifier, and a technical blender. Its versatile scent harmonizes with citrus, fruit, spice, and floral materials. Though poor in tenacity, its diffusive freshness brings lift and naturality to a broad spectrum of perfumery styles.
Synthetic Ingredient Overview
🔎 Chemical Name: 3,7-Dimethyl-1,6-octadien-3-ol
🧪 Synonyms: Licareol (L-Linalool), Coriandrol (D-Linalool), dl-Linalool
🧬 Chemical Formula: C₁₀H₁₈O
📂 CAS N°: 78-70-6
📘 FEMA: 2635
⚖️ MW: 154.25 g/mol
📝 Odor Type: Fresh, Floral
📈 Odor Strength: Medium (top note only)
👃🏼 Odor Profile: Floral-woody with a faint citrus edge; clean and light
👅 Flavor Profile: Creamy-floral, non-sweet; varies with concentration
⚗️ Uses: Top-note enhancer, floral and citrus modifier
🧴 Appearance: Clear to pale liquid
What is Linalool?
Linalool is a terpene alcohol with two chiral forms (R and S), present in over 200 plant species including lavender, rosewood, and citrus fruits. Commercially, it is available as a synthetic or semi-synthetic compound, either isolated from essential oils like Bois de Rose or synthesized via hydration of myrcene, a monoterpene from turpentine sources.
Linalool serves as a building block in perfumery and flavor chemistry, as well as a precursor for vitamin E synthesis. It is a low-boiling, top-note ingredient with limited tenacity but strong initial diffusion. The odor differs subtly between isomers: L-Linalool (from rosewood) is slightly fresher, while D-Linalool (from coriander) leans spicier.
Olfactory Profile & Perfumery Applications
Olfactory Characteristics:
Floral, slightly woody
Light citrus nuance
Subtle creamy quality in dilution
Main Perfumery Roles:
Top-note elevation in Muguet, Lavender, and Herbal bases
Used in Citrus, Fougère, Chypre, and Aromatic styles
Often included in Lilac, Sweet Pea, Apple Blossom, Frangipani, and Peony accords
Common Synergies:
Lavender, Bergamot, Geranium, Coumarin
Works well with Vanillin and citrus aldehydes
Functionality in Formula:
Imparts brightness, openness
Smooths transitions in volatile top accords
Used at 0.05%–1.5% in fine fragrance; lower in functional applications
Industrial & Technical Uses
Linalool is used widely beyond fine fragrance:
Functional Perfumes: Soaps, detergents, and air fresheners (as freshness enhancer)
Flavoring: Present in blueberry, grape, cola, and chocolate imitations
Fixative Synergy: Vanillin strengthens creamy tones and suppresses off-notes
Linalool also serves as a key intermediate in the synthesis of vitamin E and other aroma compounds (e.g., Linalyl acetate, Linalool oxides).
Regulatory & Safety Overview
IFRA: No global prohibition; QRA-based concentration limits apply depending on product category
EU Cosmetic Regulation (EC 1223/2009):
Listed as one of the 26 fragrance allergens
Declaration required above 0.001% (leave-on) and 0.01% (rinse-off)
FEMA GRAS Status: FEMA 2635
ECHA CLP Classification:
H317 — May cause allergic skin reaction
REACH registered
Phototoxicity: None known
Toxicology: Not classified as carcinogenic or mutagenic; safe under normal use
✅ Synthetic linalool is safe when used within IFRA guidelines. Natural trace impurities can influence allergenicity; purity and isomer type should be confirmed per batch.
Additional Information
Boiling Point: 198°C
Flash Point: 71°C
Density: 0.858 g/cm³ at 25°C
Solubility: Insoluble in water; soluble in ethanol and most perfume carriers
Notable Derivatives: Linalyl Acetate, Linalool Oxide, Hydroxycitronellal (via hydrogenation)
Sources
User-provided technical sheet
National Center for Biotechnology Information (PubChem CID: 6549)
IFRA 51st Amendment
Perfume and Flavor Chemicals, S. Arctander (1969)
ECHA Substance Information
FEMA GRAS Database