Technical Ingredient Overview
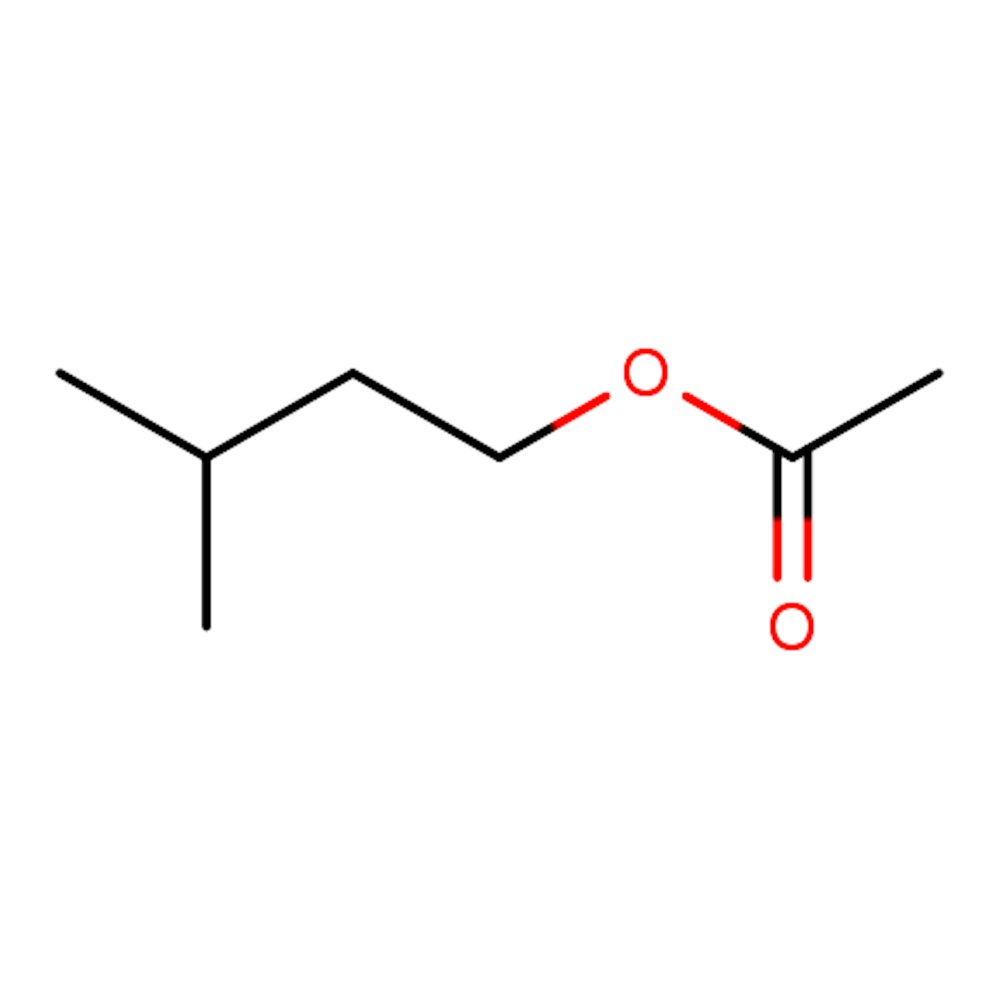
🔎 Chemical Name — 3-Methylbutyl acetate (IUPAC); Isopentyl acetate
🧪 Synonyms — Isopentyl acetate, Banana oil, Pear oil, 3-Methylbutyl ethanoate, Acetic acid 3-methylbutyl ester, Isoamyl ethanoate
📂 CAS Number — 123-92-2
📘 FEMA Number — 2055
⚖️ Molecular Weight — 130.18 g/mol
📝 Odor Type — Fruity-estery
📈 Odor Strength — Very strong; perceptible at extremely low concentrations (recommended evaluation at 1% dilution)
👃🏼 Odor Profile — Intensely sweet, banana-dominant, with juicy pear nuances, ripe apple facets, and candy-like sweetness. At higher concentrations, a sharp, solventy character reminiscent of nail varnish remover may emerge. The aroma is heady, estery, and unmistakably fruity with tropical overtones.
⚗️ Uses — Perfumery (fruity top notes, banana and pear accords, tropical compositions, jasmine enhancement, gourmand formulations); flavor industry (banana, pear, apple, strawberry, pineapple flavorings); industrial solvent (varnishes, lacquers, nitrocellulose, aircraft dope); household products (air fresheners, cleaning products); respirator fit testing agent; cosmetic formulations.
🧴 Appearance — Colorless to pale yellow liquid with low viscosity; slightly lighter than water; highly flammable.
What is Isoamyl Acetate?
Isoamyl acetate is an organic ester compound with the molecular formula C₇H₁₄O₂, formed through the esterification reaction between isoamyl alcohol (3-methylbutanol) and acetic acid. The compound belongs to the aliphatic ester family and exhibits a branched carbon chain structure that distinguishes it from its straight-chain isomer, n-amyl acetate.
This colorless liquid is only slightly soluble in water but highly soluble in most organic solvents, including alcohols and ethers. The branched molecular architecture of isoamyl acetate—specifically the methyl substituent at the third carbon position—creates its characteristic intense banana-pear aroma, which differs markedly from the milder, more generalized fruity notes of linear amyl acetate.
In perfumery terminology, isoamyl acetate is commonly referred to as "banana oil" or "pear oil," reflecting its dominant olfactory characteristics. The compound serves dual functions: as a fragrance ingredient imparting powerful fruity top notes and as an industrial solvent with excellent solvency properties for resins, oils, and nitrocellulose materials.
Historical Background
The Fischer esterification reaction—the acid-catalyzed condensation of carboxylic acids with alcohols to form esters—was first described by German chemist Emil Fischer and his collaborator Arthur Speier in 1895. Their seminal publication "Darstellung der Ester" in Chemische Berichte reported the esterification of several organic acids with absolute ethanol in the presence of reduced amounts of mineral acids (HCl or H₂SO₄), establishing the catalytic mechanism that would transform ester synthesis and become one of the most widely employed protocols in organic chemistry.
Isoamyl acetate itself was first documented by chemists in the late 1850s during systematic studies on fruit aromas, predating the Fischer-Speier mechanistic elucidation by several decades. Early organic chemists investigating natural product chemistry had identified this ester as a constituent of banana and other fruit aromas, though the efficient synthetic methodology for its preparation would not emerge until Fischer and Speier's breakthrough work demonstrated the power of acid catalysis in esterification reactions.
The discovery emerged during the golden age of organic chemistry when researchers were systematically investigating the chemical constituents responsible for natural fruit aromas. Scientists identified isoamyl acetate as occurring naturally in many plants, including apple, banana, coffee, grape, guava, lychee, papaya, peach, pomegranate, and tomato, as well as being released during fermentation processes used in making beer, sake, cognac, and whisky.
The compound found early commercial use in flavorings, confectionery, and artificial fruit essences—especially for banana and pear applications. In perfumery, it became integral to building fruity top notes and fantasy accords during the early 20th century. The material also gained industrial significance as a solvent, particularly in the formulation of nitrocellulose lacquers. As a solvent and carrier for nitrocellulose materials, isoamyl acetate was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as "aircraft dope", a critical application in early aviation before the transition to metal aircraft construction.
Throughout the 20th century, isoamyl acetate gained prominence not only for its sensory appeal but also for its pedagogical value in training olfactory professionals and chemistry students. The molecule became a teaching standard in perfumery and flavor chemistry education because its pronounced banana character makes it immediately recognizable even to novice noses. The synthesis of isoamyl acetate via Fischer esterification has become one of the most common undergraduate organic chemistry laboratory experiments, serving as an ideal representation of nucleophilic substitution and demonstrating the principles of reversible reactions and equilibrium.
Isoamyl acetate was granted Generally Recognized as Safe (GRAS) status by FEMA (Flavor and Extract Manufacturers Association) in 1965, solidifying its position as a safe and widely accepted flavoring substance. The industrial production process established in the 19th century—Fischer esterification between isoamyl alcohol and glacial acetic acid—remains essentially unchanged in its fundamental chemistry today, though modern catalysts and purification methods have improved yield and purity.
Olfactory Profile
Scent Family
Isoamyl acetate belongs to the fruity-estery scent family, representing one of the most recognizable and potent examples of aliphatic ester aroma compounds used in perfumery and flavor chemistry.
Main Descriptors
The olfactory profile of isoamyl acetate is dominated by an unmistakable, intense sweet banana character, accompanied by juicy pear nuances and hints of ripe apple. At high concentrations, the material presents overwhelming power with pure, sweet banana that can momentarily dominate compositions, resembling the concentrated essence of overripe banana peels mixed with pear-drop sweets. When a sharp solventy character emerges at elevated concentrations, it becomes reminiscent of nail varnish remover, but proper dilution reveals intensely sweet, heady fruitiness that captures the moment when tropical fruits reach peak ripeness.
The aroma can be broken down into several olfactory phases. Initially, the nose encounters an immediate burst of overripe banana sweetness with candy-like intensity. This opening impression quickly reveals secondary pear and apple facets that add juiciness and complexity to the overall fruit character. At lower concentrations, subtle estery nuances emerge that professional perfumers describe as "heady" or "reminiscent of tropical fruit at perfect ripeness." The solventy acetone-like edge that appears at higher doses becomes negligible when the material is properly diluted for fragrance work.
Intensity
The intensity of isoamyl acetate ranks among the highest of all commonly used fragrance materials. The flavor strength of isoamyl acetate is exceedingly potent and can be tasted in concentrations as low as 2 parts per million, roughly equivalent to a single drop per 50 liters. The material's remarkable olfactory potency means even trace amounts can significantly transform a composition. Professional evaluation typically requires dilution to 1% in a suitable solvent to assess its nuances without olfactory fatigue.
This exceptional intensity presents both opportunities and challenges for perfumers. On one hand, the material delivers powerful fruity impact at minimal cost and dosage. On the other hand, its dominance can easily overwhelm delicate floral notes or subtle accords if not carefully controlled. Many perfumery training programs use isoamyl acetate as a teaching example of "olfactory power" and the importance of precise measurement in formulation work.
Tenacity
Isoamyl acetate demonstrates limited tenacity in fragrance applications. The material functions as a pure top note with limited persistence, lasting approximately four hours on a blotter at full concentration. The compound flashes on quickly, sets the mood, and then steps back to allow other ingredients to take over in the fragrance development. This brief persistence stems from the compound's high volatility and low molecular weight.
For practical formulation purposes, perfumers must account for this rapid dissipation by either accepting isoamyl acetate as a fleeting opening effect or supporting it with longer-lasting materials that maintain fruity character throughout wear. Common approaches include pairing it with less volatile fruit esters (such as hexyl acetate or ethyl butyrate), incorporating fruity lactones for persistence, or building the accord on a base of fixative materials that extend the overall composition's longevity.
Volatility
Isoamyl acetate exhibits extremely high volatility. As a short-chain aliphatic ester with only seven carbon atoms, it demonstrates exceptional volatility, making it firmly positioned in the top note category of the fragrance pyramid. Unlike their parent alcohols, esters cannot act as hydrogen bond donors and consequently do not self-associate, leading to greater volatility compared to carboxylic acids of similar molecular weight. This high volatility contributes both to the material's immediate olfactory impact and its rapid dissipation from skin and textiles.
The volatility characteristics of isoamyl acetate follow predictable patterns based on its molecular structure. The branched carbon chain and relatively low molecular weight (130.18 g/mol) ensure rapid evaporation at room temperature. This physical property makes the material ideally suited for applications requiring immediate sensory impact—such as the opening spray of a perfume, the initial sniff of a scented product, or situations where a burst of recognizable fruit aroma serves a functional purpose.
Fixative Role
Isoamyl acetate does not function as a fixative material in fragrance formulations. Rather than extending the longevity of other ingredients, it requires support from longer-lasting materials to maintain its fruity character throughout fragrance development. In formulations, heavier lactones, musks, or base notes must anchor the composition to prevent the rapid loss of the banana-pear effect.
This limitation is inherent to the material's chemical structure and physical properties. Short-chain esters like isoamyl acetate cannot provide the molecular weight, low vapor pressure, or substantivity necessary for fixative action. Perfumers working with this material must therefore construct their formulas with deliberate attention to temporal balance, ensuring that as the volatile isoamyl acetate dissipates, other fruity or complementary notes emerge to maintain olfactory continuity.
Applications in Fine Fragrance
In fine fragrance composition, isoamyl acetate serves primarily as a powerful fruity top note modifier, though its use in high-end perfumery remains selective due to its overtly synthetic character at higher concentrations. The material enhances the head of a perfume and contributes fruity-banana or pear nuances, particularly in jasmine notes, though overdosing can render an accord excessively artificial.
Primary Applications
Banana and Pear Accords: Isoamyl acetate forms the olfactory backbone of straightforward banana reconstructions, often shaded with greener partners like cis-3-hexenol or enriched with creamier lactones such as γ-undecalactone for depth. When building a banana accord from scratch, perfumers typically start with isoamyl acetate as the dominant note, then add eugenol for spicy-clove nuances, vanillin for creamy sweetness, and small amounts of green notes to suggest the fresh peel character of real bananas.
Tropical Fruit Compositions: The material harmonizes with coconut, pineapple, and mango nuances, providing lift while heavier lactones and musks handle the drydown. In tropical fragrance themes—whether for fine perfumery, body care, or functional products—isoamyl acetate delivers the bright, immediately recognizable fruit character that consumers associate with vacation destinations and exotic locales. It pairs particularly well with coconut materials (coconut CO₂ extract, γ-nonalactone) and pineapple notes (allyl caproate, ethyl butyrate) to create convincing tropical fruit cocktail effects.
Jasmine Enhancement: Isoamyl acetate can be compared to benzyl propionate for adding jasmine facets. The fruity-estery character amplifies the heady, indolic aspects of jasmine absolute, creating a fuller, more radiant jasmine impression. This application technique has roots in classical French perfumery, where perfumers learned to "boost" expensive natural absolutes with carefully selected synthetic aroma chemicals. The banana-pear notes of isoamyl acetate, when used at very low levels (typically 0.1-0.3%), enhance jasmine's fruity undertones without announcing their presence as distinct banana character.
Gourmand Formulations: In dessert-inspired fragrances, isoamyl acetate contributes candy-like sweetness and fruit compote effects. The material works particularly well in compositions evoking banana splits, fruit tarts, tropical sorbets, or candy shop environments. Combined with vanilla, caramel notes (ethyl maltol, furaneol), and creamy lactones, it helps create the olfactory impression of sweet confections and desserts.
Fantasy Accords: The material supports playful, novelty scents targeting youthful demographics. Its instantly recognizable character and association with childhood candy memories make it valuable for products aimed at teenagers and young adults, particularly in body sprays, shower gels, and fun fragrance formats.
Pairing Behavior
Isoamyl acetate pairs well with ylang-ylang essential oil, coconut CO₂ extract, and jasmine materials. The material also combines effectively with other fruit esters (ethyl butyrate for pineapple, hexyl acetate for pear-apple), green alcohols (leaf alcohol, cis-3-hexenol for natural freshness), and sweet vanillic bases that round out its sharp edges. Perfumers must balance its aggressive character with softer florals or risk overwhelming delicate compositions.
Understanding these pairing relationships requires knowledge of both chemistry and sensory perception. Ylang-ylang contains naturally occurring esters that create harmonic relationships with isoamyl acetate, while also providing the floral complexity and richness that the simple ester lacks. Coconut materials share a tropical-sweet character that blends seamlessly with banana notes. Jasmine's indolic richness benefits from the bright, fruity lift that isoamyl acetate provides, while the jasmine simultaneously adds sophistication and depth to what might otherwise read as simplistic candy.
Dosage Considerations
Professional recommendations suggest limiting use to 0.1-2% in fragrance compositions to avoid artificial, candy-like effects, with a maximum recommended limit of 25% in fragrance formulas. At 0.1% the material offers a gentle pear sparkle; at 1% it becomes unmistakably banana; beyond 3% it risks overwhelming lighter florals or herbs.
These dosage guidelines reflect both the material's olfactory power and the aesthetic preferences of contemporary perfumery. Fine fragrances typically use isoamyl acetate at the lower end of this range, where it functions as a subtle modifier rather than a dominant note. Functional fragrances—household products, personal care items, and mass-market body sprays—can accommodate higher levels where bold, recognizable fruit character serves a clear purpose. The 25% maximum represents a practical upper limit beyond which the material's solventy character becomes problematic and the composition risks olfactory imbalance.
Performance in Formula
Isoamyl acetate exhibits predictable, straightforward behavior in fragrance formulations, making it a reliable workhorse material despite its intensity. The compound pours easily, behaves predictably, and rewards even small amounts with an instant banana-pear pop that livens up a blend. Laboratories appreciate its stability in typical fragrance formats, from fine perfume to household cleaners, as it maintains its character over time when stored correctly.
Solubility and Compatibility
The material demonstrates excellent solubility in alcoholic bases, oils, and most organic solvents commonly used in perfumery, including ethanol, dipropylene glycol (DPG), isopropyl myristate (IPM), and triethyl citrate. Its slight water solubility limits performance in aqueous systems but poses no practical issues in alcohol-based perfumes or oil-based formulations.
This solubility profile makes isoamyl acetate exceptionally versatile across different product formats. In alcohol-based fine fragrances, it dissolves completely and remains stable throughout the product's shelf life. In oil-based formulations such as body oils or oil perfumes, it integrates smoothly without requiring solubilizers or co-solvents. Even in products containing water phases—such as lotions or body washes where the fragrance compound exists in an emulsified state—the material performs adequately when properly formulated.
Stability Profile
Isoamyl acetate shows good oxidative stability under normal storage conditions. As an ester, it remains susceptible to hydrolysis in the presence of moisture, strong acids, or bases, which can revert the compound to its constituent alcohol and acid. Formulations should maintain neutral pH and avoid prolonged exposure to water to preserve olfactory integrity. The low flashpoint (approximately 25°C) requires careful handling during production and mandates storage away from ignition sources.
From a practical formulation standpoint, this means that perfumers can expect isoamyl acetate to maintain its banana-pear character for extended periods when stored properly—in tightly sealed containers, away from heat and light, and in environments with controlled humidity. In finished products, the material's stability depends heavily on the overall formulation pH and water content. Alcoholic perfumes with low water content provide ideal stability conditions, while products with higher water content or non-neutral pH may experience gradual ester hydrolysis over time, leading to subtle shifts in the fragrance profile.
Blending Dynamics
The material's exceptional volatility means it functions purely as a top note with limited tenacity, demanding careful support from longer-lasting materials. Perfumers often intentionally overdose isoamyl acetate knowing the opening blast will mellow rapidly once applied to skin or fabric. The compound's aggressive banana character can mask subtle nuances in complex blends during initial evaluation but dissipates quickly to reveal underlying heart and base notes.
This temporal behavior requires strategic thinking during formula development. When evaluating a new fragrance composition containing isoamyl acetate, perfumers must assess not just the immediate impression but also the fragrance's evolution over time. A formula that seems dominated by banana at the spray might reveal beautiful floral or woody facets after fifteen minutes as the isoamyl acetate volatilizes. Conversely, a formula that seems perfectly balanced on the blotter might lack sufficient opening impact when worn, necessitating an increase in top note materials including isoamyl acetate.
Technical Challenges
The primary formulation challenge lies in moderating isoamyl acetate's overwhelming intensity without losing its fruity sparkle. Predilution to 10% in ethanol, triethyl citrate, or DPG facilitates more precise weighing and safer olfactory evaluation. The material's tendency to dominate delicate florals necessitates strategic placement within the formula architecture—often best reserved for functional products or bold tropical compositions rather than refined floral bouquets.
Many professional perfumers create working solutions of isoamyl acetate at 10% concentration specifically to improve handling and dosing accuracy. When working with materials this powerful, the difference between "just right" and "too much" might be a single drop, making diluted solutions essential for precision work. Additionally, pre-dilution allows perfumers to evaluate the material's character more accurately without olfactory fatigue setting in immediately.
Industrial & Technical Uses
Beyond perfumery and flavor applications, isoamyl acetate serves important industrial functions as a versatile solvent and intermediate compound.
Solvent Applications
The material functions as an effective solvent for tannins, nitrocellulose, lacquers, celluloid, and camphor. As a solvent and carrier for nitrocellulose materials, isoamyl acetate was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as 'aircraft dope'. Today, with metal aircraft construction predominating, such use remains mostly limited to historically accurate reproductions and scale models.
The compound's solvency power stems from its ester functionality and moderate molecular weight, which allow it to dissolve both polar and moderately non-polar substances. In lacquer formulations, isoamyl acetate helps dissolve resinous materials and cellulose derivatives while evaporating at a controlled rate to leave a smooth, uniform film. Its pleasant odor makes it preferable to other industrial solvents with objectionable smells, particularly in applications where worker exposure might occur.
Chemical Synthesis
The compound serves as an intermediate in specialty chemical synthesis and participates in various organic reactions. Its ester functionality makes it useful in transesterification reactions and as a source of the isoamyl group in organic synthesis. In research laboratories, chemists use isoamyl acetate both as a reagent and as a medium for conducting organic reactions, appreciating its combination of solvency power, pleasant odor, and moderate boiling point.
Analytical and Testing Applications
Due to its intense, pleasant odor and low toxicity, isoamyl acetate is used to test the effectiveness of respirators and gas masks. This application exploits both the compound's powerful aroma—easily detected at very low concentrations—and its relatively benign toxicological profile, making it ideal for verifying proper respirator sealing without endangering test subjects.
In respirator fit testing protocols, a small amount of isoamyl acetate is released into a hood worn by the person being tested. If the respirator fits properly, the wearer should not detect the characteristic banana smell. If gaps exist in the seal between the respirator and the face, the distinctive aroma immediately signals the need for adjustment. This method provides a simple, effective, and safe way to verify respiratory protection equipment performance.
Fermentation and Biotechnology
Research explores enhancement of isoamyl acetate production through gradual supply of isoamyl alcohol during solid-state fermentation, highlighting potential in biotechnological applications. The compound represents the major ginjo-flavor component of Japanese sake (Ginjo-shu) and can be synthesized via lipase-catalyzed transesterification of ethyl acetate in n-hexane.
These biotechnological approaches represent important developments in sustainable chemical production. Rather than relying solely on petrochemical feedstocks, researchers have demonstrated that microorganisms can produce isoamyl acetate through fermentation of renewable carbohydrate sources. This opens possibilities for "natural" labeling of the ingredient and reduces dependence on fossil fuel-derived starting materials. The enzymatic synthesis routes using lipases offer mild reaction conditions and high selectivity, producing pure isoamyl acetate without the harsh catalysts and byproducts associated with traditional Fischer esterification.
Regulatory & Safety Overview
IFRA Status
Isoamyl acetate is not restricted under IFRA Amendment 51 (notified June 30, 2023) and carries no usage limitations for fragrance applications. The compound does not appear on any IFRA prohibition, restriction, or specification lists, indicating its established safety profile for use in consumer products. This regulatory status reflects decades of safe use in fragrances and the absence of documented sensitization concerns or other safety issues that would warrant restriction.
The IFRA Standards represent the fragrance industry's primary self-regulatory mechanism for ensuring safe use of fragrance ingredients. These standards are developed based on scientific risk assessments conducted by the Research Institute for Fragrance Materials (RIFM) and recommendations from an independent Expert Panel for Fragrance Safety. The fact that isoamyl acetate remains unrestricted through Amendment 51—which includes over 260 standards covering various fragrance ingredients—demonstrates its favorable safety profile.
EU Cosmetics Regulation
The material requires no mandatory allergen labeling under EU Regulation 1223/2009 (Cosmetics Regulation). Isoamyl acetate does not contain any recognized allergens according to current EU regulatory frameworks governing cosmetic ingredients.
This regulatory position is significant because the EU maintains one of the world's most stringent cosmetic ingredient regulatory frameworks. The EU Cosmetics Regulation requires labeling of 26 fragrance allergens when present above specific thresholds, and prohibits use of substances identified as sensitizers or otherwise problematic. The absence of isoamyl acetate from these lists confirms its acceptability for use in cosmetic products marketed throughout the European Union.
FEMA Status
FEMA Number 2055; the compound was granted Generally Recognized as Safe (GRAS) status by FEMA in 1965. As a synthetic flavoring substance, isoamyl acetate is permitted for direct addition to food for human consumption, provided it is used in the minimum quantity required to produce its intended effect and in accordance with all principles of good manufacturing practice.
The GRAS designation represents the U.S. Food and Drug Administration's recognition that qualified experts consider the substance safe for its intended use. This status, established in 1965 and maintained through subsequent reviews, allows isoamyl acetate to be used in food products without requiring pre-market approval as a food additive. The longevity of this GRAS status—nearly six decades—further underscores the material's safety record.
Usage Levels in Products
Reported concentrations in various products include: non-alcoholic beverages (28 ppm), ice cream and ices (56 ppm), candy (190 ppm), baked goods (120 ppm), gelatins and puddings (100 ppm), and chewing gum (2,700 ppm). In fragrance applications, typical concentrations are: soap (0.05%), detergent (0.005%), creams and lotions (0.003%), and perfume (0.05%).
These usage levels provide useful benchmarks for formulators working across different product categories. The substantially higher concentration permitted in chewing gum (2,700 ppm or 0.27%) compared to beverages (28 ppm or 0.0028%) reflects both the material's safety profile and the different sensory requirements of various applications. Chewing gum requires concentrated flavoring to deliver persistent taste during mastication, while beverages need only sufficient flavoring to create the desired taste experience in dilute aqueous solution.
Toxicology
Isoamyl acetate exhibits low toxicity, contributing to its widespread acceptance in consumer products. The Priority-based Assessment of Food Additives (PAFA) database reports possible average daily intake of isoamyl acetate from foods at 24.49 mg. The National Occupational Exposure Survey conducted by NIOSH between 1981 and 1983 estimated that 97,668 employees, including 32,512 female workers in 24 industries, were potentially exposed to isoamyl acetate.
This toxicological profile indicates that isoamyl acetate poses minimal health risks under normal use conditions. The average daily dietary intake of approximately 25 mg represents a very small exposure level, well below any threshold of toxicological concern. Occupational exposure data from the NIOSH survey helps establish that even workers who regularly handle the material in industrial settings have not demonstrated adverse health effects, supporting its safe use designation.
Environmental Profile
Isoamyl acetate is readily biodegradable and poses low ecotoxicological risk when used in fragrance applications, according to European Chemicals Agency (ECHA) assessments. The compound can be produced via bio-based fermentation routes (e.g., yeast fermentation of carbohydrates), offering sustainable sourcing potential for green formulators.
The ready biodegradability of isoamyl acetate means that when released into the environment—whether through wastewater from manufacturing facilities, consumer use of fragranced products, or other pathways—microorganisms in soil and water can break down the compound into harmless substances relatively quickly. This characteristic, combined with low aquatic toxicity, means isoamyl acetate does not bioaccumulate in organisms or persist in the environment as a pollutant. For formulators developing products with environmental sustainability goals, these properties make isoamyl acetate a responsible choice among fruity aroma chemicals.
Production and Sourcing
Synthetic Production
Isoamyl acetate is prepared through Fischer esterification, the acid-catalyzed reaction between isoamyl alcohol and glacial acetic acid, typically using sulfuric acid as catalyst. Alternatively, p-toluenesulfonic acid or an acidic ion exchange resin can serve as the catalyst. The commercial-grade material is also produced by rectification of commercial amyl acetate.
The Fischer esterification process proceeds through a well-understood mechanism. In the presence of an acid catalyst, the carbonyl carbon of acetic acid becomes electrophilic, allowing attack by the nucleophilic oxygen of isoamyl alcohol. Through a series of proton transfers and elimination of water, the ester bond forms. Because this reaction is reversible and reaches equilibrium, manufacturers typically employ techniques to drive the reaction to completion: using excess acetic acid, removing water as it forms (often through azeotropic distillation), or both. The crude ester undergoes purification through distillation to remove unreacted starting materials, water, and catalyst residues, yielding high-purity isoamyl acetate.
The isoamyl alcohol feedstock originates from two primary sources: fractional distillation of fusel oil (a byproduct of alcoholic fermentation containing mixed higher alcohols), or petrochemical synthesis routes. The Fischer esterification reaction combining isoamyl alcohol with glacial acetic acid under acidic conditions proves efficient and cost-effective, yielding high-purity ester upon distillation.
Fusel oil, the traditional source of isoamyl alcohol, represents an excellent example of valorizing industrial byproducts. During production of ethanol for beverages or fuel, fermentation of starches and sugars produces not only ethanol but also small amounts of higher alcohols (propanol, butanol, isoamyl alcohol, and others). These congener alcohols concentrate in the "heads" and "tails" fractions during distillation and are collectively termed fusel oil. Rather than discarding this material, the chemical industry purifies it to recover individual alcohols including isoamyl alcohol, which then serves as feedstock for ester production.
Natural Occurrence and Biosynthesis
Isoamyl acetate occurs naturally in bananas, apples, and other fruits, where it is biosynthesized via enzymatic esterification of isoamyl alcohol and acetic acid. The compound is released by fermentation processes, including those used for making beer, sake, cognac, and whisky. During fermentation, isoamyl acetate is formed by an enzyme called acetate transferase, with temperature control playing a crucial role—higher temperatures (21-24°C) promote acetate formation.
The natural biosynthesis of isoamyl acetate in fruits occurs through plant metabolic pathways that produce both the precursor alcohol and the activated acetic acid derivative (acetyl-CoA), which then combine through enzymatic catalysis. This process contributes to fruit ripening and the development of characteristic fruit aromas. In bananas, isoamyl acetate concentration increases dramatically during ripening, contributing to the fruit's signature smell that signals optimal eating maturity.
In fermented beverages, yeast metabolism produces isoamyl acetate as a secondary metabolite. The alcohol transferase enzymes in yeast catalyze formation of various esters from alcohols and acyl-CoA compounds. Brewers and winemakers manipulate fermentation conditions—particularly temperature and yeast strain selection—to control ester production and achieve desired flavor profiles. Weissbeers and Belgian ales, for example, deliberately promote higher ester formation including isoamyl acetate to create their characteristic fruity character, while lager brewers typically ferment at lower temperatures to minimize ester production and achieve cleaner, crisper flavor profiles.
Interestingly, isoamyl acetate is released by a honey bee's sting apparatus where it serves as a pheromone beacon to attract other bees and provoke them to sting, demonstrating the compound's biological significance beyond its fruit-derived origins. This alarm pheromone function in Apis mellifera represents one of nature's chemical communication systems, where the volatile ester signals danger to other members of the colony.
Market Position
Thanks to wide demand and straightforward production, isoamyl acetate sits at the inexpensive end of the raw-material spectrum, allowing perfumers to use it freely in both luxury and mass-market formulas. Specific production volume data indicates 112,000 pounds were produced in the United States in 1984, with fragrance usage estimated at approximately 10,000 pounds per year in the early 1970s.
This favorable cost position stems from multiple factors: abundant availability of starting materials (fusel oil from fermentation industries or petrochemical-derived isoamyl alcohol), efficient and well-established synthetic routes, and economies of scale from high production volumes serving both flavor and fragrance markets. The material's low cost removes economic barriers to its use, meaning formulators can make creative decisions based purely on olfactory considerations rather than budgetary constraints.
References
Arctander, S. (1960). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
Burdock, G. A. (2010). Fenaroli's Handbook of Flavor Ingredients (6th ed.). CRC Press.
European Chemicals Agency (ECHA). (2024). Isoamyl acetate – Substance Information. Retrieved from https://echa.europa.eu
Fischer, E., & Speier, A. (1895). Darstellung der Ester. Chemische Berichte, 28, 3252-3258. https://doi.org/10.1002/cber.189502803176
Howard, P. H. (1990). Handbook of Environmental Fate and Exposure Data for Organic Chemicals, Vol. II. Chelsea, MI: Lewis Publishers, Inc.
Opdyke, D. L. J. (1975). Monographs on fragrance raw materials. Food and Cosmetics Toxicology, 13(Suppl.), 551.
Rowan, D. D. (2011). Volatile metabolites. In Annual Plant Reviews: Fruit and Seed Production (Vol. 42, pp. 1–39). Wiley-Blackwell.
Sell, C. S. (2019). The Chemistry of Fragrances (3rd ed.). Royal Society of Chemistry.
Smith, B., March, J., & Carey, F. A. (2006). March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.). Wiley-Interscience.
U.S. National Toxicology Program (NTP). (n.d.). Summary of Data for Chemical Selection: Isoamyl Acetate CAS No. 123-92-2. Retrieved from https://ntp.niehs.nih.gov