Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Globalide 100 (Habanolide)
Premium Synthetic Ingredient for Perfumery
Globalide® (Habanolide) is a synthetic macrocyclic musk classified as a 15-membered unsaturated ketone. Developed for modern fragrance applications, it delivers a soft, metallic-powdery musk effect with exceptional tenacity on fabric and skin. Its olfactory signature bridges white-musk top notes and lactonic base materials, making it valuable in both fine fragrance and functional perfumery.
Commercially available as Globalide® 100 by Symrise, it is prized for its high purity, linear macrocyclic structure, and strong blooming effect in textiles and alcohol-based systems.
Premium Synthetic Ingredient for Perfumery
Globalide® (Habanolide) is a synthetic macrocyclic musk classified as a 15-membered unsaturated ketone. Developed for modern fragrance applications, it delivers a soft, metallic-powdery musk effect with exceptional tenacity on fabric and skin. Its olfactory signature bridges white-musk top notes and lactonic base materials, making it valuable in both fine fragrance and functional perfumery.
Commercially available as Globalide® 100 by Symrise, it is prized for its high purity, linear macrocyclic structure, and strong blooming effect in textiles and alcohol-based systems.
Premium Synthetic Ingredient for Perfumery
Globalide® (Habanolide) is a synthetic macrocyclic musk classified as a 15-membered unsaturated ketone. Developed for modern fragrance applications, it delivers a soft, metallic-powdery musk effect with exceptional tenacity on fabric and skin. Its olfactory signature bridges white-musk top notes and lactonic base materials, making it valuable in both fine fragrance and functional perfumery.
Commercially available as Globalide® 100 by Symrise, it is prized for its high purity, linear macrocyclic structure, and strong blooming effect in textiles and alcohol-based systems.
Technical Ingredient Overview
🏭 Manufacturer — Symrise AG (trade name Globalide® 100)
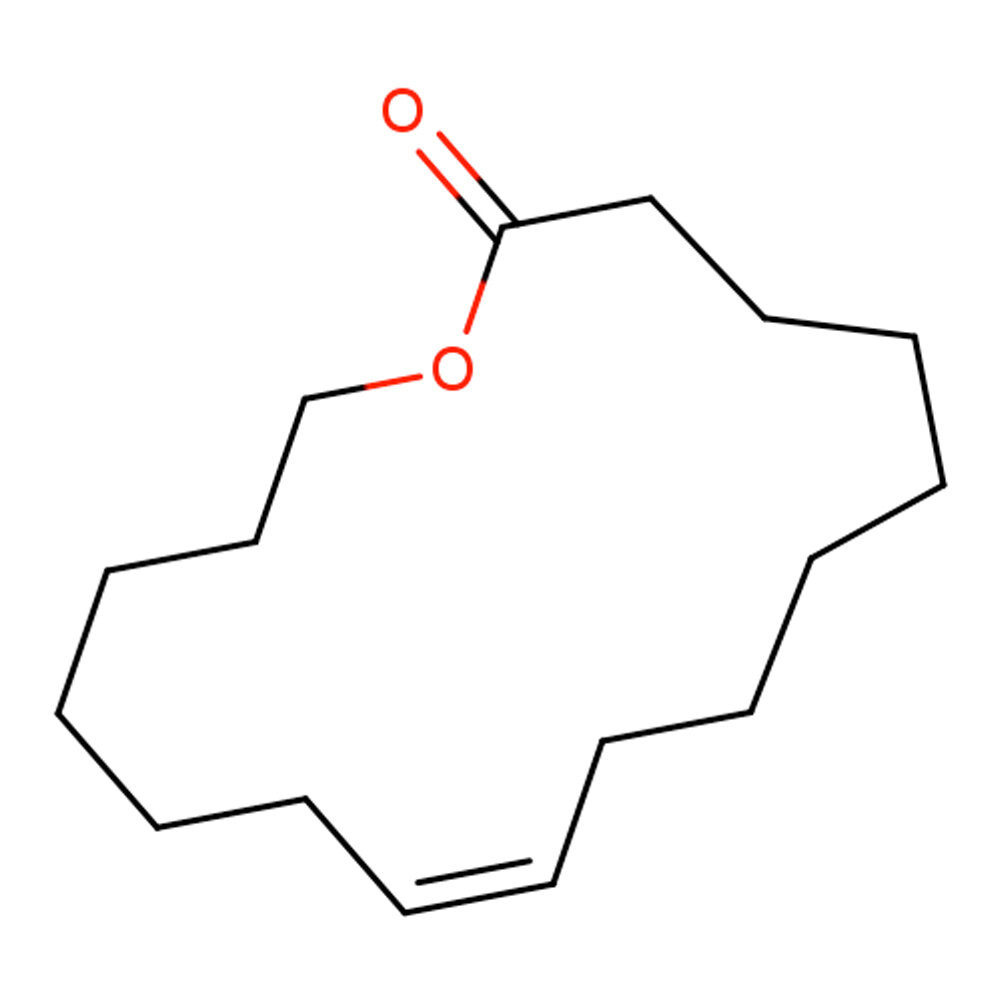
🔎 Chemical Name — (12E)-oxacyclohexadec-12-en-2-one
🧪 Synonyms — Globalide®, Habanolide®, Oxacyclohexadecenone, Musk Habanolide
🧬 Chemical Formula — C₁₅H₂₆O₂
📂 CAS — 34902-57-3 (EU); 111879-80-2 (US TSCA)
📘 FEMA — 4889
⚖️ MW — 238.37 g · mol⁻¹
📝 Odor Type — Macrocyclic musk (metallic “hot-iron” nuance)
📈 Odor Strength — Medium diffusion; > 14 days substantivity on paper blotter
👃🏼 Odor Profile — Elegant, powdery-clean, soft-warm (the ambery-er among the musks), faintly woody-metallic
⚗️ Uses — Fine-fragrance base notes, fabric- & hair-care, detergent musk boosters, candle blends
🧴 Appearance — Colourless to very pale-yellow oily liquid, low volatility at r.t.
What is Globalide® / Habanolide?
Globalide® (Habanolide) is a 15-membered unsaturated macrocyclic ketone musk in the “linear-macrocyclic” sub-class. Its ring system combines the radiance of alicyclic musks with the soft elegance of long-chain lactones, yielding a high-impact yet nuanced fixative in modern perfumery (PubChem, 2025; Firmenich, 2024).
Historical Background
The molecule was first described in 1969 during Firmenich’s macrocyclic-musk program (Arctander, 1969). Large-scale manufacture became feasible only after 1990 with the advent of catalytic ring-closing metathesis (RCM) routes (BenchChem, 2024). Symrise introduced its optically pure grade as Globalide® 100 in 2002, providing a cost-efficient alternative to Firmenich’s Habanolide® (Symrise, 2002). RCM—pioneered by Grubbs—remains the industrial synthesis of choice owing to high atom economy and E-selectivity (Wikipedia, 2025).
Olfactory Profile & Performance
Scent family. Musky-powdery (macrocyclic musk)
Descriptors. Clean linen, soft powder, warm metal, lightly woody
Intensity & Tenacity. Medium initial diffusion; outstanding substantivity (> 336 h on fabric)
Volatility (GC RI ≈ 2700). Late heart → base note; evaporation half-life ≈ 35 h
Fixative role. Bridges metallic “white-musk” top notes (e.g. Helvetolide) with heavier lactones such as Exaltolide, extending overall dry-down by ~ 15–20 %.
Applications in Fine Fragrance
Globalide® is dosed 0.1–3 % in contemporary colognes to impart a “clean ironed-cotton” effect and build volume in white-floral bouquets (e.g., Narciso Rodriguez For Her, 2003) (Perfumer & Flavorist, 2004). It shows notable synergy with Muscénone®, Ambrettolide, and Ethylene Brassylate, often replacing polycyclic musks in eco-label formulations.
Behaviour in Formula
The molecule withstands alkaline soap processing, exhibits minimal discoloration, and is stable to UVC ≤ 254 nm. At ≥ 0.5 % it boosts blooming in alcohol and micro-encapsulated fabric softeners; however, aquatic toxicity (see below) necessitates effective wastewater treatment.
Industrial & Technical Uses
Beyond prestige perfumery, Globalide® is valued as a high-impact, readily biodegradable musk for premium detergents, hair conditioners, and scented candles where metal-powdery freshness and long-lasting substantivity are required. Its low odor threshold (~ 0.01 µg · L⁻¹ air) permits use at < 0.05 % in consumer cleaners while remaining IFRA-compliant.
Regulatory & Safety Overview
IFRA 51: No specific concentration limits for Categories 1–12 (IFRA, 2023).
GHS / CLP: Classified Aquatic Chronic 1, H410 “Very toxic to aquatic life with long-lasting effects”.
EU Cosmetic Allergen List (Annex III): Not listed among the 26 declarable fragrance allergens (SCCS, 2012).
REACH: Registered; no harmonised hazard classification beyond environmental endpoints (ECHA, 2024).
FEMA / GRAS: FEMA No. 4889 — permitted as flavouring but seldom used owing to metallic after-taste.
RIFM Safety: “No sensitisation concern; systemic NOAEL = 150 mg · kg⁻¹ d⁻¹ (rat, 90 d)” (Api et al., 2017).
Good manufacturing practice recommends tailored wastewater treatment to prevent chronic aquatic exposure (Scentspiracy, 2025).
Additional Information
Compatibility. Excellent with aldehydic musks and ionones; avoid high levels (> 5 %) with intensely fruity lactones that can mask its metallic facet.
Natural occurrence. Not found in nature; fully synthetic.
Chirality. Commercial grades are optically enriched to the (S)-enantiomer, enhancing diffusivity relative to the racemate (Symrise, 2002
Sources
pi, A. M., Belsito, D., Botelho, D., et al. (2017). RIFM fragrance-ingredient safety assessment: Oxacyclohexadecane-2,13-dione (CAS 38223-29-9). Food and Chemical Toxicology, 110, S711–S717.
Arctander, S. (1969). Perfume and Flavor Chemicals (Aroma Chemicals). Montclair, NJ: Author.
BenchChem. (2024). Oxacyclohexadec-12-en-2-one product brief. Retrieved April 28, 2025, from https://www.benchchem.com
Firmenich. (2024). HABANOLIDE® product information sheet. DSM-Firmenich internal publication.
International Fragrance Association. (2023). IFRA Certificate of Conformity: HABANOLIDE® 947303. IFRA.
Perfumer & Flavorist. (2004). Perfumery: Techniques in evolution, Part V. Perfumer & Flavorist, 29(5), 54–63.
PubChem. (2025). Oxacyclohexadecenone (CID 12410118). National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov
Symrise. (2002). Globalide® 100 technical dossier [Internal report].
Wikipedia. (2025). Ring-closing metathesis. Retrieved April 28, 2025, from https://en.wikipedia.org