Technical Ingredient Overview
🏭 Manufacturer —IFF
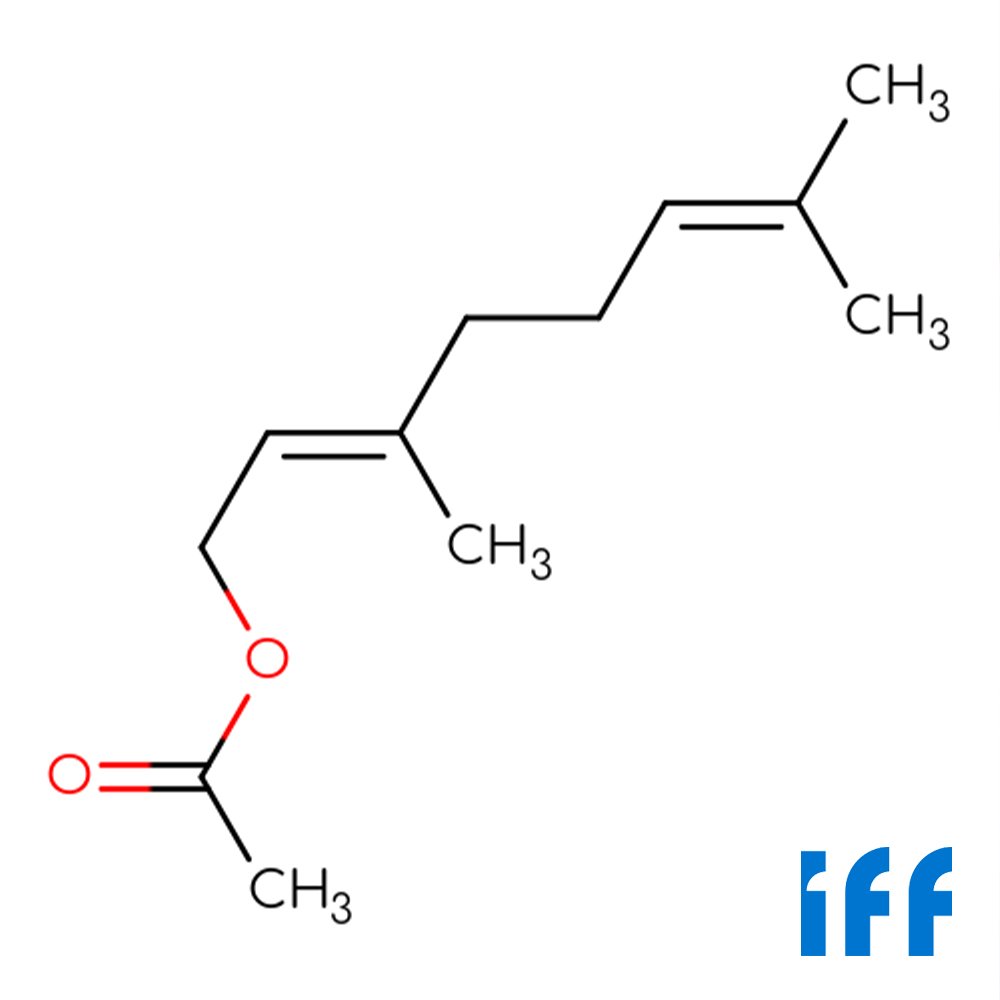
🔎 Chemical Name — (2E)-3,7-Dimethylocta-2,6-dien-1-yl acetate
🧪 Synonyms — trans-3,7-Dimethyl-2,6-octadien-1-yl acetate; Geraniol acetate; Acetic acid geraniol ester; trans-Geranyl acetate; Geranyl ethanoate; (E)-3,7-Dimethyl-2,6-octadien-1-yl acetate
📂 CAS Number — 105-87-3
📘 FEMA Number — 2509 (JECFA 58)
⚖️ Molecular Weight — 196.29 g/mol
📝 Odor Type — Floral-fruity
📈 Odor Strength — Medium to medium-strong
👃🏼 Odor Profile — Fresh, rosy-floral with pronounced fruity nuances reminiscent of pear, accompanied by subtle lavender and citrus undertones; sweet, waxy floral character with metallic-rose aspects (due to neryl acetate content); green, slightly penetrating quality with creamy body
⚗️ Uses — Rose, lavender, geranium, neroli, and citrus compositions in fine and functional perfumery; floral modifiers in soap, detergent, and personal care formulations; GRAS-approved flavoring agent in foods and beverages (citrus, berry, tropical fruit flavors); essential oil reconstitutions
🧴 Appearance — Colorless to pale yellow oily liquid; density (d₂₀) ≈ 0.9080–0.9160 g/cm³; refractive index (n₂₀/D) ≈ 1.461–1.464; insoluble in water, freely soluble in ethanol and organic solvents
What is Geranyl Acetate?
Geranyl Acetate is an acyclic monoterpenoid ester formed through the acetylation of geraniol, a primary terpene alcohol. Chemically classified as an unsaturated aliphatic ester, it belongs to the geranyl ester family within the broader monoterpene class (Burdock, 2016; Surburg & Panten, 2006). The molecule features a trans-configured double bond at the 2-position and contains two isoprene units characteristic of monoterpenes, with the acetate functional group conferring enhanced volatility and diffusion properties compared to its parent alcohol.
Commercial geranyl acetate typically contains 1-2% of its geometric isomer neryl acetate (the cis-isomer), which contributes a subtle metallic nuance to the overall fragrance profile (Arctander, 1960). This natural co-occurrence reflects the isomeric composition found in essential oils where both esters are present. The compound exhibits characteristic monoterpene reactivity, including susceptibility to acid-catalyzed rearrangements and oxidative degradation in alkaline conditions (Sell, 2006).
Historical Background
The synthesis of geranyl acetate represents one of the earliest deliberate explorations of structure-odor relationships in organic chemistry. In 1869, Ferdinand Tiemann and his colleagues at the University of Berlin successfully acetylated natural geraniol isolated from palmarosa oil, producing geranyl acetate and establishing its structural identity (Arctander, 1960; Sell, 2006). This pioneering work formed part of Tiemann's broader investigation into terpene chemistry and laid foundational principles for synthetic fragrance chemistry.
During the late 19th century, geranyl acetate remained a specialty material obtained primarily through fractional distillation of essential oils rich in the ester, particularly Eucalyptus and Callitris species oils (containing up to 60%), palmarosa oil (up to 14%), and smaller quantities in geranium, citronella, and lavender oils (Surburg & Panten, 2006). The compound's commercial significance expanded dramatically post-1945 when industrial-scale terpene production from turpentine and related feedstocks made synthetic geraniol economically viable, enabling efficient esterification routes to geranyl acetate (Bauer et al., 2001).
The development of large-scale Fischer esterification processes using geraniol and acetic acid or acetic anhydride transformed geranyl acetate into a high-volume fragrance ingredient by the mid-20th century. Today, it ranks among the most widely used terpene esters in both fragrance and flavor industries, with several hundred tons produced annually (Surburg & Panten, 2006). High-purity grades designated "Geranyl Acetate Extra" are supplied by major fragrance houses for fine perfumery applications, while natural fractions continue to serve the natural products market (IFF, 2024).
Olfactory Profile
Scent Family: Floral (rose subfamily) with fruity-green modifiers
Main Descriptors: The olfactory character of geranyl acetate is dominated by a fresh, rosy-floral quality with distinctive fruity nuances, particularly reminiscent of ripe pear. The scent profile exhibits complexity: an initial sweet, fruity-rosy impression with soft citrus undertones evolves into a creamy, waxy floral body. The presence of neryl acetate (typically 1-2%) imparts an unusual freshness and subtle metallic-rose character with faint citronella aspects (Arctander, 1960). Compared to pure geraniol, the acetate demonstrates smoother, more rounded floral tones with enhanced fruity sweetness and reduced sharpness. Secondary descriptors include green, lavender-like, and slightly penetrating qualities.
Intensity: Medium to medium-strong odor strength. Geranyl acetate possesses good diffusivity and projects well from formulations without overwhelming other components. Detection threshold ranges from 9 to 460 ppb depending on matrix (Sigma-Aldrich, 2024).
Tenacity: Moderate persistence. As a top-to-middle note ingredient, geranyl acetate demonstrates an evaporation half-life of approximately 2 hours at 20°C on a smelling strip, placing it in the middle volatility range. While less tenacious than base note materials, it provides reasonable substantivity in formulations and contributes to the heart phase of fragrance development.
Volatility: Moderate volatility characteristic of monoterpene esters. Boiling point at 1.5 kPa is 98°C; at 101.3 kPa approximately 230–240°C (Surburg & Panten, 2006). Vapor pressure at 20°C is approximately 0.07 mmHg. Functions primarily as a top-to-middle note with bias toward middle notes due to its acetate functionality.
Fixative Role: Limited fixative properties. While not employed primarily as a fixative, geranyl acetate can contribute to bridging volatility gaps between highly volatile top notes (such as citral) and less volatile floral middle notes. Its moderate evaporation rate helps smooth out the transition between fragrance phases and can extend the life of more ephemeral citrus notes.
Applications in Fine Fragrance
Geranyl acetate functions as a versatile floral modifier and fruity-rosy accent in fine perfumery, particularly valued for its ability to round sharp aldehydic notes and contribute natural-feeling rose and lavender effects. The material serves multiple compositional roles:
Rose Compositions: Essential component in rose reconstructions where it adds fruity depth and softness. Pairs synergistically with geraniol, citronellol, phenylethyl alcohol, and damascones to build natural, full-bodied rose accords. Typical usage: 0.5–2% of concentrate (Arctander, 1960).
Lavender and Fougère: Contributes to authentic lavender profiles alongside linalyl acetate, providing fruity-sweet modulation. Used in fougère structures to add floral-fruity complexity.
Citrus and Cologne: Valuable in citrus compositions for smoothing sharp citral notes and adding floral complexity. Particularly effective in bergamot, neroli, and petitgrain accords. Often combined with neryl acetate at equal ratios in lemon compositions to soften synthetic citral harshness (Perfumer & Flavorist, 2024).
Fruity-Floral Bouquets: Natural fit in peach, pear, apple, and tropical fruit fragrances where its pear-like nuance reinforces fruity themes while maintaining floral elegance.
Soap and Detergent Perfumery: Widely used in functional fragrance for household products due to cost-effectiveness and performance. Note that partial hydrolysis occurs in high-pH soap bases, with approximately 60% conversion to geraniol after 24 hours; this can be mitigated with antioxidants and pH stabilizers.
Typical Dosage: 0.1–2% in fine fragrance concentrates; up to 12% in functional fragrance bases (Perfumer's Apprentice, 2024).
Performance in Formula
Solubility and Stability: Highly soluble in ethanol, diethyl phthalate, and common fragrance solvents. Practically insoluble in water. Exhibits good stability under neutral pH conditions but undergoes hydrolysis in alkaline environments, liberating geraniol and acetic acid. In soap formulations, retention decreases to approximately 60% after 24 hours due to saponification reactions.
Blending Behavior: Demonstrates excellent blending characteristics with most fragrance materials. Particularly synergistic with other monoterpene alcohols and esters (geraniol, citronellol, linalool, linalyl acetate, neryl acetate), creating smooth, natural-feeling rose and floral-fruity accords. Functions as an effective bridge between highly volatile citrus top notes and less volatile floral hearts, smoothing out volatility gaps. The material's moderate diffusion rate ensures even development throughout the fragrance life cycle.
Compositional Role: Acts primarily as a modifier and smoother rather than a dominant character note. Rounds sharp aldehyde notes (particularly citral), softens synthetic aspects in compounded florals, and adds fruity nuances to enhance naturalness. Geranyl acetate is indispensable for creating specific nuances in rose, lavender, geranium, and citrus reconstructions (Surburg & Panten, 2006).
Technical Considerations: Protect from light to prevent photo-oxidation. Store in cool conditions (2–8°C recommended for long-term storage). Avoid contact with strong oxidizing agents. In alkaline formulations, consider using stabilizers or accepting the transformation to geraniol as a design feature.
Industrial & Technical Uses
Food and Beverage Flavoring: GRAS-approved (FEMA 2509) for use in foods and beverages. Contributes to citrus (lemon, lime, orange, grapefruit), berry (strawberry, raspberry, blackberry), tropical fruit, rose, neroli, and Earl Grey tea flavors. Typical usage: 200–2000 ppm in flavor concentrates intended for 0.05% dosage in finished beverages (Perfumer & Flavorist, 2024). Taste characteristics at 20 ppm described as green, floral, fruity with citrus nuances.
Fragrance Manufacturing: Used as an intermediate and raw material in the production of blended rose, lavender, and geranium bases for downstream fragrance compounding.
Essential Oil Reconstitution: Important constituent in the reconstitution and standardization of natural essential oils including geranium, citronella, palmarosa, petitgrain, and lavender oils.
Personal Care and Cosmetics: Incorporated into formulations for shampoos, conditioners, body lotions, soaps, and other toiletries to impart fresh, floral-fruity fragrances.
Household Products: Widely used in detergents, fabric softeners, surface cleaners, and air fresheners for functional fragrance applications.
Regulatory & Safety Overview
IFRA Status: Geranyl acetate is not subject to restrictions or prohibitions under the IFRA Standards up to and including the 51st Amendment (effective 2023–2025). The material may be used without quantitative restrictions in fragrance compounds across all product categories 1–12 (IFRA, 2024). Not listed in the IFRA Standards Index as a restricted or prohibited material.
EU Cosmetics Regulation: Compliant with Regulation (EC) No 1223/2009 and subsequent amendments. Not listed in Annexes II (prohibited substances), III (restricted substances), or IV (colorants) of the EU Cosmetics Regulation. CAS 105-87-3 is permitted for use in cosmetic products without specific restrictions. EC Number: 203-341-5.
FEMA Status: Designated FEMA 2509 with GRAS (Generally Recognized As Safe) status for use as a flavoring agent in foods and beverages. JECFA Number: 58. Evaluated and affirmed safe at current intake levels by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1998). Listed in 21 CFR 182.60 (U.S. Code of Federal Regulations) (FEMA, 2024).
Toxicology: The safety assessment of geranyl acetate has been comprehensively evaluated by regulatory bodies and industry safety panels. National Toxicology Program (NTP) gavage studies in F344/N rats and B6C3F1 mice using food-grade geranyl acetate (71% geranyl acetate, 29% citronellyl acetate) found no evidence of carcinogenicity (NTP, 1987). Recent research indicates mildly toxic effects by ingestion at high doses and identifies the material as a human skin irritant; mutation data have been reported in some studies (Sigma-Aldrich, 2024). The compound is classified as Skin Irritant Category 2 and Skin Sensitizer Category 1 under GHS classification, requiring appropriate handling precautions in occupational settings. However, at use levels encountered in consumer products, geranyl acetate demonstrates an excellent safety profile with no safety concern at current intake levels (JECFA assessment).
Handling and Safety: Combustible liquid. Avoid strong oxidizing agents. Use appropriate personal protective equipment when handling neat material. Well-ventilated work areas recommended. Environmental classification: Aquatic Chronic Category 3 (Sigma-Aldrich, 2024).
References
Arctander, S. (1960). Perfume and Flavor Materials of Natural Origin (pp. 300-302).
European Chemicals Agency. (2024). Geranyl acetate – Substance information (EC 203-341-5).
European Commission. (2023). Regulation (EU) 2023/1545 amending Annex III of Regulation (EC) 1223/2009.
Flavor & Extract Manufacturers Association. (2025). Geranyl acetate (FEMA 2509).
International Flavors & Fragrances. (2025). Geranyl Acetate Extra – Ingredient Compendium.
International Fragrance Association. (2024). IFRA Transparency List.
PubChem. (2025). Geranyl acetate (CID 1549026). National Center for Biotechnology Information.
Sigma-Aldrich. (2024). Safety Data Sheet – Geranyl acetate.
Sell, C. S. (2006). The Chemistry of Fragrances (2nd ed.). Royal Society of Chemistry.