Technical Ingredient Overview
🏭 Manufacturer — IFF (International Flavors & Fragrances)
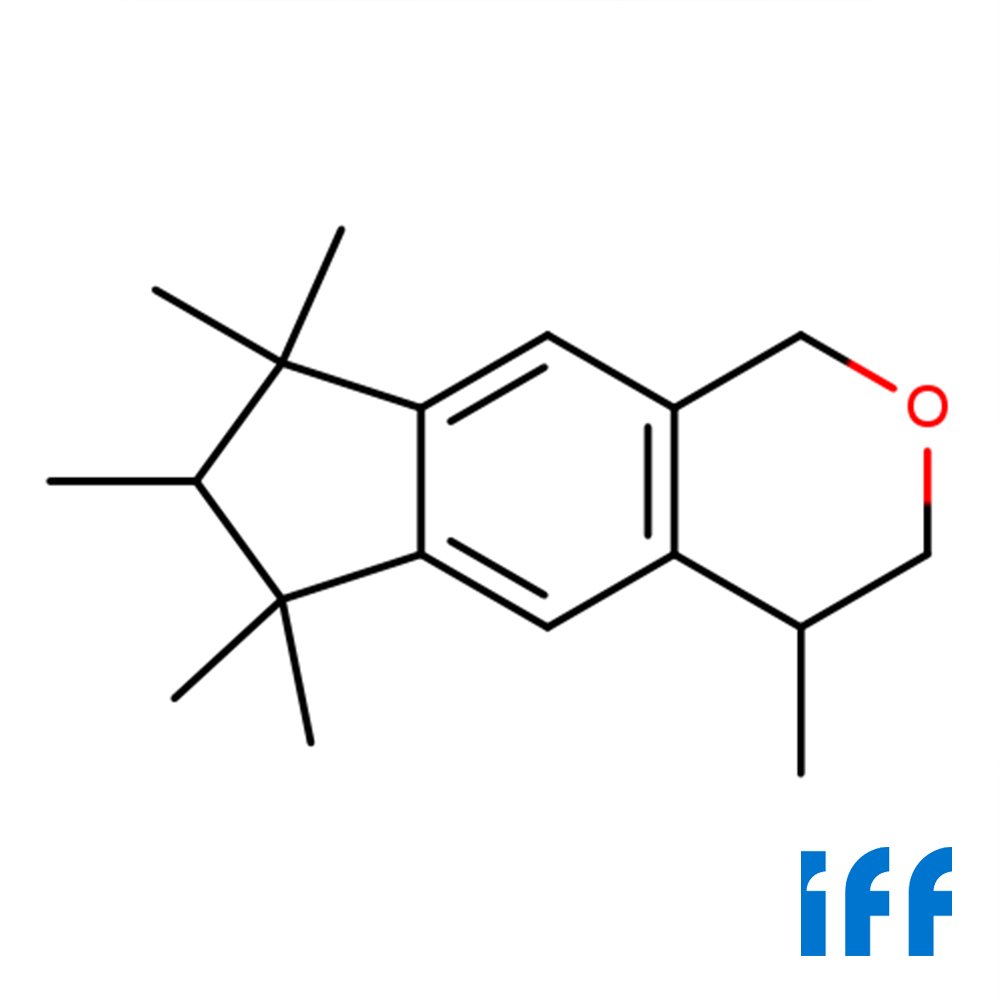
🔎 Chemical Name — 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran
🧪 Synonyms — Galaxolide, HHCB, Hexamethylindanopyran
🧬 Chemical Formula — C₁₈H₂₆O
📂 CAS — 1222-05-5
📘 FEMA — not applicable
⚖️ MW — 258.4 g mol⁻¹
📝 Odor Type — Musk
📈 Odor Strength — Medium → strong
👃🏼 Odor Profile — Sweet, clean, musky, woody, subtly floral
⚗️ Uses — Fine fragrance, personal-care, fabric-care, home-care
🧴 Appearance — Clear to pale-yellow liquid
What is Galaxolide?
Galaxolide is a synthetic polycyclic musk celebrated for its clean, sweet-musky character, exceptional stability, and high substantivity. Introduced by IFF in the mid-1960s, it offered perfumers an ethical, cost-effective replacement for scarce animal musk without sacrificing olfactory richness (Sell, 2019).
Historical Background
1960 – 1969: Early development and launch
Synthesised at IFF by chemists Lambert G. Heeringa and Martin G. J. Beets; their work was patented in 1967 (Heeringa & Beets, 1967). Commercial release as Galaxolide® followed in 1965, giving industry its first major polycyclic musk.1970 – 1980s: Global market expansion
Adoption spread from fine perfumery to detergents and fabric softeners; by 1988 annual output exceeded 6 000 t, making Galaxolide one of the world’s highest-tonnage aroma chemicals (Sell, 2019).1990 – 2000: Environmental awareness
Monitoring programmes detected polycyclic musks in surface waters and aquatic organisms. Peck and Hornbuckle (2004) found Galaxolide at ng L⁻¹ levels in Lake Michigan, demonstrating trophic transfer. Laboratory and field work reported a bioconcentration factor of roughly 600–1 600 and log Kₒw ≈ 5.5, indicating persistence (Rimkus, 1999).2001 – 2010: Formal risk assessments
The EU Scientific Committee on Cosmetic Products (SCCNFP, 2002) deemed Galaxolide safe for cosmetics. A comprehensive EU risk-assessment and subsequent SCHER opinion (2008) concluded no additional risk-reduction was necessary. IFRA introduced voluntary caps, codified as category-specific limits in the 51st Amendment (IFRA, 2024).2011 – 2025: Contemporary perspective
The U.S. EPA listed Galaxolide as a high-priority TSCA substance and issued an aquatic-risk assessment citing moderate persistence and chronic toxicity (EPA, 2015). Australia’s NICNAS/IMAP programme (NICNAS, 2021) reached similar conclusions while judging current exposure manageable. EU consumption has fallen ≈ 35 % between 2005 and 2023 as formulators pivot to more biodegradable macrocyclic musks; nonetheless, Galaxolide remains among the five most-used musks worldwide.
Olfactory Profile
Scent family: Musk
Main descriptors: Sweet, clean, musky, woody, soft floral nuance
Intensity: Medium–strong
Tenacity: Very high (≥ 24 h on skin, ≥ 1 week on paper)
Volatility: Low; functions chiefly as a base note and fixative
Applications in Fine Fragrance
Galaxolide’s versatility allows usage from trace amounts up to 10 % of a concentrate.
For compositions seeking a modern, airy musk-woody effect, Galaxolide blends exceptionally well with Iso E Super, enhancing both the diffusion and the creamy depth of the base notes.
To add a sensual, creamy texture to musky compositions, Delta-Decalactone serves as a perfect complement to Galaxolide, enriching its warm and velvety nuances.
When paired with luminous floral ingredients like Benzyl Acetate, Galaxolide reinforces the soft, petal-like qualities of the heart accord, providing a clean and polished musk finish.
In gourmand accords where a sweet musky background is desired, Ethyl Maltol can synergize with Galaxolide, enhancing the addictive, cozy facets of the fragrance.
Typical roles include white-musk bases, clean-laundry dry-downs, and soft-woody florals.
Performance in Formula
Galaxolide blooms smoothly, lending volume and diffusion while remaining compatible with esters, alcohols, aldehydes, lactones, and nitriles. It keeps odour integrity from pH 4 to 10 and up to 180 °C (e.g., deodorant processing). Above ≈ 5 % it may overshadow very delicate tea-floral facets.
Industrial & Technical Uses
Galaxolide provides long-lasting “clean laundry” notes in detergents (50–300 ppm), enduring skin scent in body-care, heat-stable musk in candles, and malodour-masking freshness in air-care aerosols.
Regulatory & Safety Overview
IFRA 51st Amendment (2024): Category 4 leave-on products limited to 1.5 %; higher limits for rinse-off categories.
GHS: Not classified for acute human toxicity; Aquatic Chronic 2.
EU Cosmetics Regulation: Permitted; not among the 26 declarable fragrance allergens.
REACH: Registered; not formally PBT under Annex XIII.
Key environmental data: BCF 600–1 600; log Kₒw ≈ 5.5 (Rimkus, 1999).
EPA TSCA: High-priority substance; 2015 assessment recommends monitoring aquatic exposure (EPA, 2015).
Additional Information
Galaxolide is detectable in wastewater effluents and sediments at ng–µg kg⁻¹ but is significantly reduced by advanced tertiary treatment. Industry is gradually transitioning to more biodegradable macrocyclic musks such as ambrettolide and exaltolide.
References
EPA. (2015). TSCA work-plan chemical risk assessment: HHCB (CAS 1222-05-5). U.S. Environmental Protection Agency.
European Commission Scientific Committee on Health and Environmental Risks. (2008). Opinion on the risk-assessment report on 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran (HHCB).
Heeringa, L. G., & Beets, M. G. J. (1967). Perfume compound and process of making the same (U.S. Patent No. 3 360 530). United States Patent and Trademark Office.
International Flavors & Fragrances. (n.d.). Galaxolide ingredient compendium. Retrieved April 28, 2025, from https://www.iff.com/scent/ingredients-compendium/galaxolide
International Fragrance Association. (2024). IFRA standards – 51st amendment.
NICNAS. (2021). IMAP human health tier II assessment: 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran (HHCB). National Industrial Chemicals Notification and Assessment Scheme, Australian Government.
Peck, A. M., & Hornbuckle, K. C. (2004). Synthetic musk fragrances in Lake Michigan. Environmental Science & Technology, 38(2), 367–372. https://doi.org/10.1021/es034769y
Rimkus, G. G. (1999). Polycyclic musk fragrances in the aquatic environment. Toxicology Letters, 111(1–2), 37–56. https://doi.org/10.1016/S0378-4274(99)00195-0
SCCNFP. (2002). Opinion concerning HHCB adopted by the Scientific Committee on Cosmetic Products and Non-Food Products. European Commission.
Sell, C. S. (2019). The chemistry of fragrances: From perfumer to consumer (3rd ed.). Royal Society of Chemistry.