 Image 1 of 2
Image 1 of 2

 Image 2 of 2
Image 2 of 2



Galaxolide ®
Premium Synthetic Ingredient for Perfumery
Galaxolide is a synthetic polycyclic musk developed by IFF in the 1960s, chemically classified as a substituted iso-chroman. Recognized for its clean, sweet-floral musk character, it bridges the tonal gap between polycyclic and macrocyclic musks, delivering exceptional oxidative stability and high diffusion.
With moderate impact and extreme persistence (lasting up to 400 hours on paper), Galaxolide is widely used across fine fragrance and functional perfumery applications.
It is available in pure form or as a 50% dilution in Dipropylene Glycol (DPG) to facilitate accurate dosing and easier handling.
Premium Synthetic Ingredient for Perfumery
Galaxolide is a synthetic polycyclic musk developed by IFF in the 1960s, chemically classified as a substituted iso-chroman. Recognized for its clean, sweet-floral musk character, it bridges the tonal gap between polycyclic and macrocyclic musks, delivering exceptional oxidative stability and high diffusion.
With moderate impact and extreme persistence (lasting up to 400 hours on paper), Galaxolide is widely used across fine fragrance and functional perfumery applications.
It is available in pure form or as a 50% dilution in Dipropylene Glycol (DPG) to facilitate accurate dosing and easier handling.
Premium Synthetic Ingredient for Perfumery
Galaxolide is a synthetic polycyclic musk developed by IFF in the 1960s, chemically classified as a substituted iso-chroman. Recognized for its clean, sweet-floral musk character, it bridges the tonal gap between polycyclic and macrocyclic musks, delivering exceptional oxidative stability and high diffusion.
With moderate impact and extreme persistence (lasting up to 400 hours on paper), Galaxolide is widely used across fine fragrance and functional perfumery applications.
It is available in pure form or as a 50% dilution in Dipropylene Glycol (DPG) to facilitate accurate dosing and easier handling.
Technical Ingredient Overview
🏭 Manufacturer — IFF (International Flavors & Fragrances)
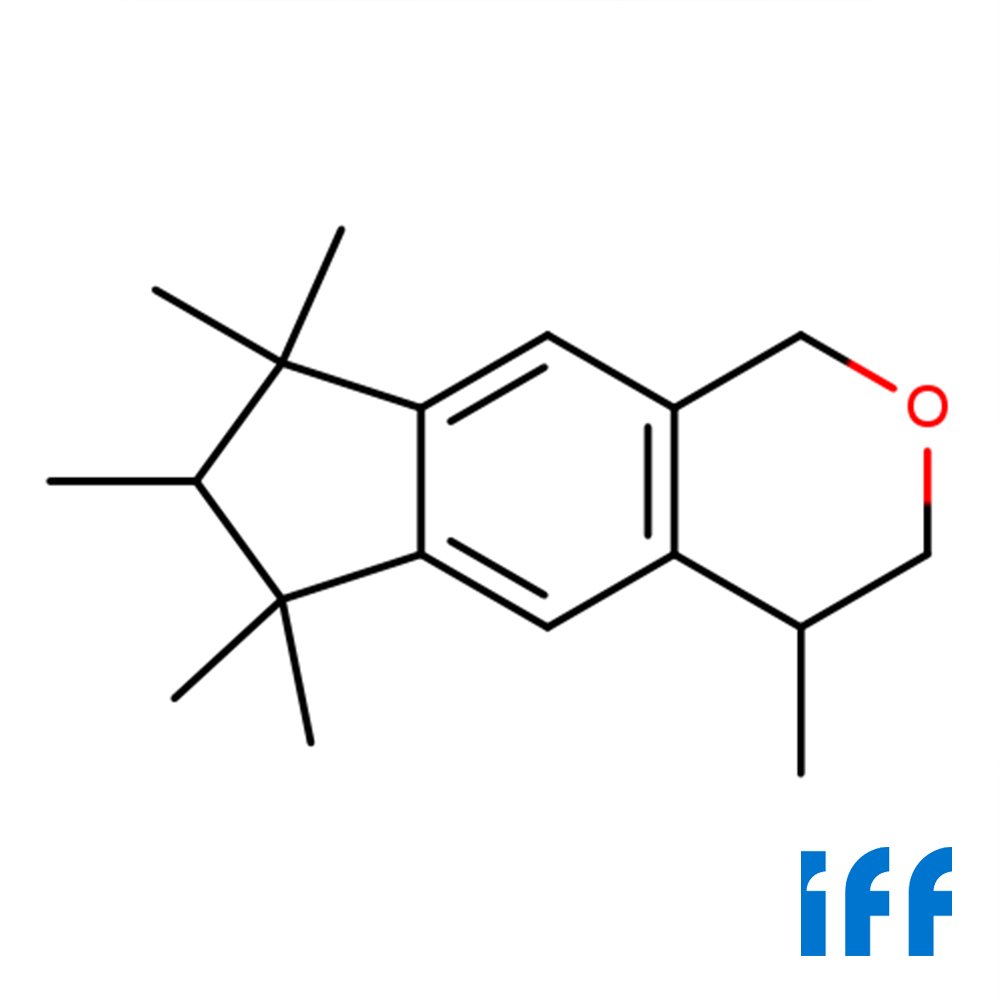
🔎 Chemical Name — 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran
🧪 Synonyms — Galaxolide, HHCB, Hexamethylindanopyran
🧬 Chemical Formula — C₁₈H₂₆O
📂 CAS — 1222-05-5
📘 FEMA — not applicable
⚖️ MW — 258.4 g mol⁻¹
📝 Odor Type — Musk
📈 Odor Strength — Medium → strong
👃🏼 Odor Profile — Sweet, clean, musky, woody, subtly floral
⚗️ Uses — Fine fragrance, personal-care, fabric-care, home-care
🧴 Appearance — Clear to pale-yellow liquid
What is Galaxolide?
Galaxolide is a synthetic polycyclic musk celebrated for its clean, sweet-musky character, exceptional stability, and high substantivity. Introduced by IFF in the mid-1960s, it offered perfumers an ethical, cost-effective replacement for scarce animal musk without sacrificing olfactory richness (Sell, 2019).
Historical Background
1960 – 1969: Early development and launch
Synthesised at IFF by chemists Lambert G. Heeringa and Martin G. J. Beets; their work was patented in 1967 (Heeringa & Beets, 1967). Commercial release as Galaxolide® followed in 1965, giving industry its first major polycyclic musk.1970 – 1980s: Global market expansion
Adoption spread from fine perfumery to detergents and fabric softeners; by 1988 annual output exceeded 6 000 t, making Galaxolide one of the world’s highest-tonnage aroma chemicals (Sell, 2019).1990 – 2000: Environmental awareness
Monitoring programmes detected polycyclic musks in surface waters and aquatic organisms. Peck and Hornbuckle (2004) found Galaxolide at ng L⁻¹ levels in Lake Michigan, demonstrating trophic transfer. Laboratory and field work reported a bioconcentration factor of roughly 600–1 600 and log Kₒw ≈ 5.5, indicating persistence (Rimkus, 1999).2001 – 2010: Formal risk assessments
The EU Scientific Committee on Cosmetic Products (SCCNFP, 2002) deemed Galaxolide safe for cosmetics. A comprehensive EU risk-assessment and subsequent SCHER opinion (2008) concluded no additional risk-reduction was necessary. IFRA introduced voluntary caps, codified as category-specific limits in the 51st Amendment (IFRA, 2024).2011 – 2025: Contemporary perspective
The U.S. EPA listed Galaxolide as a high-priority TSCA substance and issued an aquatic-risk assessment citing moderate persistence and chronic toxicity (EPA, 2015). Australia’s NICNAS/IMAP programme (NICNAS, 2021) reached similar conclusions while judging current exposure manageable. EU consumption has fallen ≈ 35 % between 2005 and 2023 as formulators pivot to more biodegradable macrocyclic musks; nonetheless, Galaxolide remains among the five most-used musks worldwide.
Olfactory Profile
Scent family: Musk
Main descriptors: Sweet, clean, musky, woody, soft floral nuance
Intensity: Medium–strong
Tenacity: Very high (≥ 24 h on skin, ≥ 1 week on paper)
Volatility: Low; functions chiefly as a base note and fixative
Applications in Fine Fragrance
Galaxolide’s versatility allows usage from trace amounts up to 10 % of a concentrate.
For compositions seeking a modern, airy musk-woody effect, Galaxolide blends exceptionally well with Iso E Super, enhancing both the diffusion and the creamy depth of the base notes.
To add a sensual, creamy texture to musky compositions, Delta-Decalactone serves as a perfect complement to Galaxolide, enriching its warm and velvety nuances.
When paired with luminous floral ingredients like Benzyl Acetate, Galaxolide reinforces the soft, petal-like qualities of the heart accord, providing a clean and polished musk finish.
In gourmand accords where a sweet musky background is desired, Ethyl Maltol can synergize with Galaxolide, enhancing the addictive, cozy facets of the fragrance.
Typical roles include white-musk bases, clean-laundry dry-downs, and soft-woody florals.
Performance in Formula
Galaxolide blooms smoothly, lending volume and diffusion while remaining compatible with esters, alcohols, aldehydes, lactones, and nitriles. It keeps odour integrity from pH 4 to 10 and up to 180 °C (e.g., deodorant processing). Above ≈ 5 % it may overshadow very delicate tea-floral facets.
Industrial & Technical Uses
Galaxolide provides long-lasting “clean laundry” notes in detergents (50–300 ppm), enduring skin scent in body-care, heat-stable musk in candles, and malodour-masking freshness in air-care aerosols.
Regulatory & Safety Overview
IFRA 51st Amendment (2024): Category 4 leave-on products limited to 1.5 %; higher limits for rinse-off categories.
GHS: Not classified for acute human toxicity; Aquatic Chronic 2.
EU Cosmetics Regulation: Permitted; not among the 26 declarable fragrance allergens.
REACH: Registered; not formally PBT under Annex XIII.
Key environmental data: BCF 600–1 600; log Kₒw ≈ 5.5 (Rimkus, 1999).
EPA TSCA: High-priority substance; 2015 assessment recommends monitoring aquatic exposure (EPA, 2015).
Additional Information
Galaxolide is detectable in wastewater effluents and sediments at ng–µg kg⁻¹ but is significantly reduced by advanced tertiary treatment. Industry is gradually transitioning to more biodegradable macrocyclic musks such as ambrettolide and exaltolide.
References
EPA. (2015). TSCA work-plan chemical risk assessment: HHCB (CAS 1222-05-5). U.S. Environmental Protection Agency.
European Commission Scientific Committee on Health and Environmental Risks. (2008). Opinion on the risk-assessment report on 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran (HHCB).
Heeringa, L. G., & Beets, M. G. J. (1967). Perfume compound and process of making the same (U.S. Patent No. 3 360 530). United States Patent and Trademark Office.
International Flavors & Fragrances. (n.d.). Galaxolide ingredient compendium. Retrieved April 28, 2025, from https://www.iff.com/scent/ingredients-compendium/galaxolide
International Fragrance Association. (2024). IFRA standards – 51st amendment.
NICNAS. (2021). IMAP human health tier II assessment: 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-γ-2-benzopyran (HHCB). National Industrial Chemicals Notification and Assessment Scheme, Australian Government.
Peck, A. M., & Hornbuckle, K. C. (2004). Synthetic musk fragrances in Lake Michigan. Environmental Science & Technology, 38(2), 367–372. https://doi.org/10.1021/es034769y
Rimkus, G. G. (1999). Polycyclic musk fragrances in the aquatic environment. Toxicology Letters, 111(1–2), 37–56. https://doi.org/10.1016/S0378-4274(99)00195-0
SCCNFP. (2002). Opinion concerning HHCB adopted by the Scientific Committee on Cosmetic Products and Non-Food Products. European Commission.
Sell, C. S. (2019). The chemistry of fragrances: From perfumer to consumer (3rd ed.). Royal Society of Chemistry.