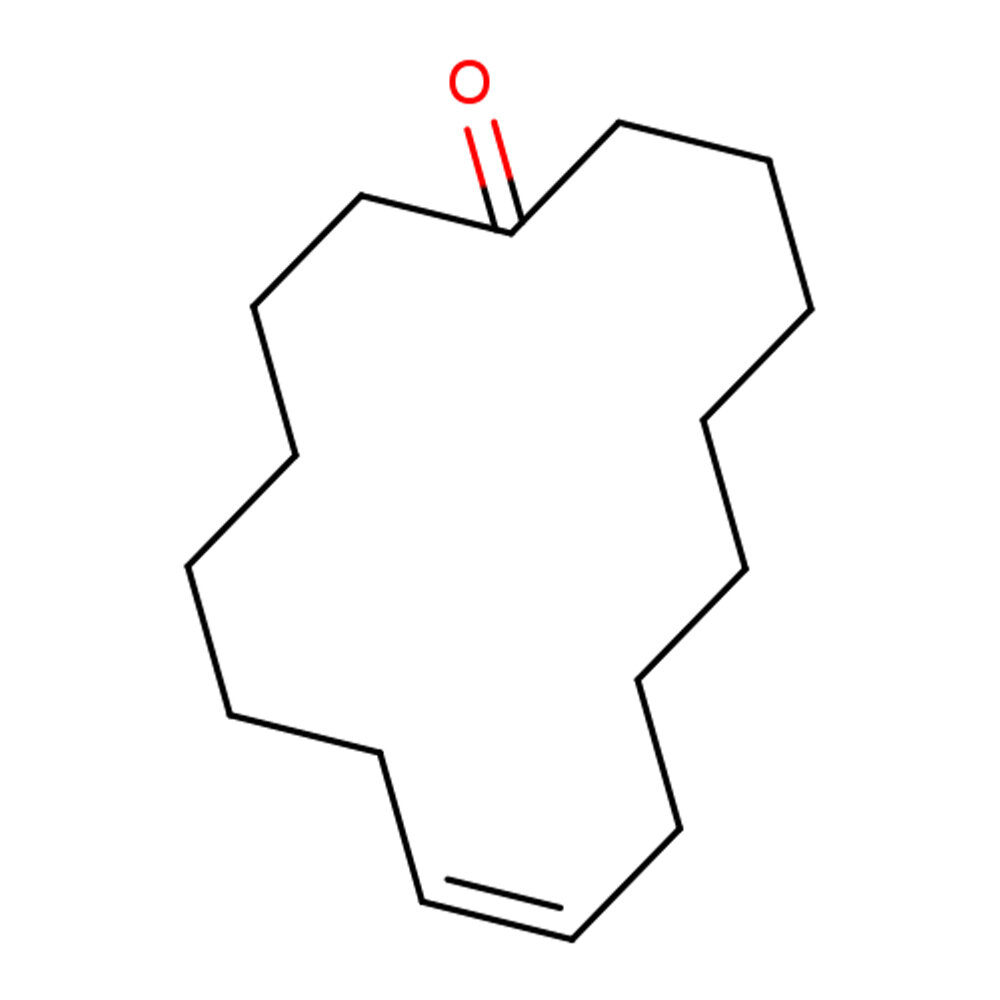
Musk T (Ethylene Brassylate) TECHNICAL INGREDIENT OVERVIEW
🔎 Chemical Name — 1,4-Dioxacycloheptadecane-5,17-dione
🧪 Synonyms — Astrotone, Astratone, Ethylene Glycol Brassylate, Brassylic Acid Ethylene Ester, Ethylene Tridecanedioate, Ethylene Undecane Dicarboxylate, MC-5, Musk NN, Emeressence 1150, Cyclo-1,13-ethylenedioxytridecan-1,13-dione, Tridecanedioic Acid Cyclic Ethylene Glycol Diester
📂 CAS Number — 105-95-3
📘 FEMA Number — 3543 (GRAS status for flavoring applications)
📘 EINECS Number — 203-347-8
⚖️ Molecular Weight — 270.36–270.37 g/mol
🧬 Molecular Formula — C₁₅H₂₆O₄
📝 Odor Type — Macrocyclic Musk
📈 Odor Strength — Medium intensity; exceptional tenacity exceeding 200 hours on blotter
👃🏼 Odor Profile — Soft, clean, sweet musk with warm, powdery, floral character; subtle vanilla nuances; faint red fruit and ambrette-like aspects; creamy, slightly oily undertone
⚗️ Uses — Fixative in fine fragrances, base note enhancer, substantivity booster, musk accord builder, soft sensual facet contributor
🧴 Appearance — Colorless to pale yellow clear viscous liquid
🌡️ Physical Properties —
Boiling Point: 138–142°C at 133 Pa; 330–434°C at atmospheric pressure
Flash Point: >100°C (212°F)
Density/Specific Gravity: 1.040–1.049 g/mL at 20–25°C
Refractive Index: 1.468–1.473 at 20°C
Vapor Pressure: <0.001 mm Hg at 20°C (negligible)
Water Solubility: Practically insoluble (~1.7 mg/L at 25°C)
Log P (octanol/water): 4.3–4.7 (highly lipophilic)
What is Musk T (Ethylene Brassylate)?
Musk T, chemically known as ethylene brassylate, is a synthetic macrocyclic musk belonging to the class of macrolides and analogues. It is a 17-membered cyclic diester formed by the cyclization of brassylic acid (1,13-tridecanedioic acid) with ethylene glycol. This compound represents a key member of the macrocyclic musk family, characterized by a large lactone ring structure that mimics the olfactory properties of natural animal musks while offering superior stability, consistency, and ethical sourcing compared to animal-derived materials.
As a macrocyclic lactone, ethylene brassylate features two ester linkages within its ring structure, contributing to its distinctive sweet, powdery, and lactonic nuances that differentiate it from macrocyclic ketones such as muscone. The compound's conformational flexibility and low polarity enable it to interact effectively with olfactory receptors associated with musk perception, producing a smooth, diffusive aroma with exceptional persistence yet subtle character.
Ethylene brassylate is classified as a "white musk" and functions primarily as a base note ingredient in perfumery due to its extremely low evaporation rate and high molecular weight. It does not occur naturally in significant quantities and is exclusively produced through synthetic chemical processes.
Historical Background
Ethylene brassylate was first synthesized in 1933–1934 by chemists at E.I. du Pont de Nemours and Company (DuPont) in the United States, marking a significant milestone in synthetic musk chemistry. The compound emerged during a pivotal period in fragrance chemistry when the industry was transitioning from nitro musks—discovered by Albert Baur in 1888—toward safer and more stable alternatives.
The late 19th and early 20th centuries saw important developments in musk synthesis. Following Baur's nitro musks (Musk Ketone, Musk Xylene), Leopold Ruzicka achieved a breakthrough in the 1920s by successfully synthesizing macrocyclic musks like civetone and muscone from natural precursors, proving that large ring structures could replicate natural musk odors. Ruzicka's pioneering work, which later contributed to his Nobel Prize in Chemistry, established the foundation for macrocyclic musk chemistry. Companies like Firmenich introduced Exaltolide® (15-pentadecanolide) in 1927–1930, and Ruzicka introduced Exaltone® (cyclopentadecanone) in 1926 at the extraordinary price of 50,000 CHF/kg due to low synthetic yields.
DuPont's innovation with ethylene brassylate involved cyclizing brassylic acid with ethylene glycol to form a macrocyclic diester, creating a new musk molecule that was initially produced under limited license as a captive ingredient. The company marketed it under trade names including "Musk T" (possibly denoting "Tonkin" musk substitute) and "Astrotone."
The perfumer André Fraysse is credited as an early adopter who recognized the material's potential immediately upon its introduction. Around 1934, Fraysse incorporated ethylene brassylate into the reformulation of Lanvin's iconic aldehydic floral masterpiece Arpège (originally launched in 1927), adding it to the musk fond (base accord) to enhance the powdery, musky drydown. This marked the ingredient's debut in fine fragrance at a time when Lanvin was expanding to industrial proportions.
Ethylene brassylate gained rapid popularity due to several practical advantages: it was a liquid at room temperature (unlike solid musks like Exaltolide®), making it easy to handle and incorporate into formulations; it was considerably cheaper than other non-nitro musks of the early 20th century such as muscone and civetone; and it offered excellent fixative properties and olfactory versatility.
The availability of synthetic macrocyclic musks like ethylene brassylate culturally influenced perfumery's evolution during the mid-20th century, enabling a shift toward cleaner, softer musk bases and moving away from the heavy animalic notes favored in 19th-century perfumes. By the 1950s and beyond, as concerns about the toxicity and environmental persistence of nitro musks grew, macrocyclic musks like Musk T became increasingly important to the industry.
Olfactory Profile
Scent Family
Macrocyclic Musk — classified as a "white musk" type
Main Descriptors
Ethylene brassylate presents a soft, clean, and sweet musk character with warm, creamy, and slightly powdery qualities that provide a velvety sensation to fragrance compositions. The odor profile includes:
Primary character: Soft musky, sweet, powdery
Secondary facets: Floral (subtle), fruity (faint red fruit nuance), ambrette-like aspects, vanilla-like sweetness
Tertiary nuances: Woody, spicy undertones, slight oily quality
Overall impression: Warm, sensual, silky, diffusive yet subtle
The presence of two ester functional groups within the macrocyclic ring imparts a sweeter, more lactonic and powdery character compared to macrocyclic ketones. These ester linkages contribute to the faint fruity-vanilla aspects, as esters typically convey sweetness in olfactory perception.
Compared to polycyclic musks like Galaxolide, ethylene brassylate is less diffusive and projects more softly, but it compensates with superior tenacity and a more delicate, refined character. Some perfumers describe it as having a vanillic note in the drydown, distinguishing it from other musks in the macrocyclic family.
Intensity
Medium odor strength with a discernible olfactory impact typically starting below 1% concentration in perfume bases. The compound has an odor threshold estimated in the low nanogram per liter range (specific threshold data varies by source). Despite moderate initial intensity, its exceptional substantivity means the scent persists strongly over time.
Tenacity
Exceptional longevity exceeding 200 hours on blotter, making it one of the most persistent fragrance materials available. This extreme tenacity stems from its high molecular weight (270.36 g/mol), very low vapor pressure, and high lipophilicity (log P 4.3–4.7). The compound exhibits outstanding substantivity on skin and textiles.
Volatility
Base note classification with an extremely low evaporation rate. As a heavy macrocyclic molecule, ethylene brassylate remains in the drydown phase of fragrances, providing long-lasting musky warmth and depth. Its negligible vapor pressure (<0.001 mm Hg at 20°C) ensures it persists through all phases of fragrance development.
Fixative Role
Ethylene brassylate functions as an excellent fixative, slowing the evaporation of more volatile top and middle notes while contributing its own substantive musk character. It enhances overall fragrance longevity and helps bind together diverse olfactory components. The material provides a soft, sensual foundation that supports and extends floral, oriental, fruity, and woody accords without dominating the composition.
Applications in Fine Fragrance
Ethylene brassylate serves as a versatile and essential building block in modern perfumery, particularly valued for its ability to provide long-lasting base notes and enhance overall composition tenacity. Its applications include:
Fixative function: Extends fragrance longevity across all product categories
Musk accords: Foundational component in clean, white musk bases
Floral compositions: Supports rose, jasmine, ylang-ylang, and other floral notes with soft musky warmth
Oriental fragrances: Contributes depth and sensuality to amber, vanilla, and spice accords
Fruity florals: The red fruit nuance creates synergy with berry, peach, and tropical fruit notes
Chypre and fougère: Adds modern musk character to classic structures
Accord synergies: Blends exceptionally well with ethyl linalool, supporting both top note retention and base note persistence
Ethylene brassylate performs well in combination with other musks and is often paired with materials such as Zenolide (another cost-effective macrocyclic musk), Galaxolide, and Tonalide to create complex, multi-dimensional musk effects. It is particularly effective in compositions requiring a soft vanillic note in the drydown.
Performance in Formula
Typical concentration ranges: 0.2–10% in fragrance concentrates, with most formulas using 0.5–3%. The material has a discernible impact below 1% and can be used at higher levels (up to 10%+) for specific effects, though typical usage remains modest. IFRA imposes no quantitative restrictions, theoretically allowing up to 100% use.
Stability: Exceptionally stable in alcoholic solutions and functional fragrances. Maintains performance in soap, detergents, and alkaline applications without discoloration. However, lower-grade materials may develop fatty-rancid, castor oil-like off-notes upon aging.
Solubility: Insoluble in water; soluble in ethanol and most organic solvents; poorly soluble in propylene glycol.
Handling: Liquid at room temperature, facilitating easy incorporation into formulas without heating or special preparation.
Industrial & Technical Uses
Flavor applications: FEMA GRAS status (FEMA #3543) permits use in food flavoring at trace concentrations, contributing vanilla-musky profiles to baked goods, frozen dairy, soft candy, gelatin, puddings, and beverages. Typical use level: up to 280 ppm.
Consumer products: Widely used in shampoos, body washes, lotions, creams, household cleaners, laundry detergents, and fabric softeners for long-lasting scent and fixative effects.
Polymer chemistry: Poly(ethylene brassylate) (PEB) is a biodegradable polyester with poly(ε-caprolactone)-like properties, produced through polymerization. The compound can be depolymerized to yield the musk material.
Regulatory & Safety Overview
IFRA Status: No restrictions under IFRA Amendment 51 (effective June 30, 2023). Ethylene brassylate is permitted at 100% concentration in all product categories with no quantitative limits, indicating excellent safety profile. IFRA Standards Library
EU Cosmetics Regulation 1223/2009: Compliant; not listed among the 26 declarable fragrance allergens. No reproductive or endocrine concerns noted under REACH.
FEMA Status: GRAS (Generally Recognized as Safe) for food flavoring use; FEMA #3543.
Toxicology: RIFM safety assessment confirms low toxicity profile with no genotoxicity, skin sensitization, or reproductive/developmental toxicity concerns (McGinty et al., 2011; Api et al., 2016). Patch test studies found no sensitization issues. Not classified as PBT (Persistent, Bioaccumulative, Toxic) or vPvB (very Persistent, very Bioaccumulative) under REACH. Exhibits low bioaccumulation and favorable biodegradability compared to nitro and polycyclic musks.
Environmental profile: Macrocyclic musks like ethylene brassylate are environmentally favorable, with low bioaccumulation potential and generally higher biodegradability than polycyclic or nitro musks. Worldwide production volume exceeds 1,000 metric tons annually.
References
Api, A. M., Belsito, D., Bruze, M., Cadby, P., Calow, P., Dagli, M. L., Dekant, W., Ellis, G., Fryer, A. D., Fukayama, M., Griem, P., Hickey, C., Kromidas, L., Lalko, J. F., Liebler, D. C., Miyachi, Y., Politano, V. T., Renskers, K., Ritacco, G., Salvito, D., Schultz, T. W., Sipes, I. G., Smith, B., Vitale, D., & Wilcox, D. K. (2016). RIFM fragrance ingredient safety assessment, ethylene brassylate, CAS Registry Number 105-95-3. Food and Chemical Toxicology, 97, S71–S81. https://doi.org/10.1016/j.fct.2016.09.018
Arctander, S. (1969). Perfume and flavor chemicals (aroma chemicals) (Vol. 1). Steffen Arctander.
Kraft, P., Bajgrowicz, J. A., Denis, C., & Fráter, G. (2000). Odds and trends: Recent developments in the chemistry of odorants. Angewandte Chemie International Edition, 39(17), 2980–3010. https://doi.org/10.1002/1521-3773(20000901)39:17<2980::AID-ANIE2980>3.0.CO;2-#
McGinty, D., Letizia, C. S., & Api, A. M. (2011). Fragrance material review on ethylene brassylate. Food and Chemical Toxicology, 49(Suppl 2), S149–S156. https://doi.org/10.1016/j.fct.2011.07.011
National Center for Biotechnology Information. (2025). PubChem Compound Summary for CID 61014, 1,4-Dioxacycloheptadecane-5,17-dione. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/61014
Rowe, D. J. (Ed.). (2005). Chemistry and technology of flavors and fragrances. Blackwell Publishing.
Sell, C. S. (Ed.). (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Royal Society of Chemistry.
Internal Cross-References:
See The Musks: An Insight for classification and usage differences among musk types
Related materials: Zenolide, Galaxolide, Tonalide, Exaltolide, Muscone, Civetone