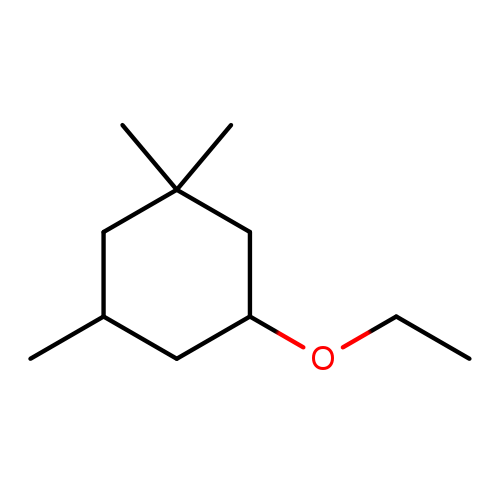
Dimethyl Acetaldehyde Phenyl Acetal (PADMA) Technical Ingredient Overview
🔎 Chemical Name — Phenylacetaldehyde dimethyl acetal
🧪 Synonyms — PADMA, Viridine®, (2,2-Dimethoxyethyl)benzene, 1,1-Dimethoxy-2-phenylethane, Rosal, Vert de Lilas, Vertodor, Veracetal, Foliacetal, α-Tolylaldehyde dimethyl acetal, Benzene (2,2-dimethoxyethyl)-, 2,2-Dimethoxy-1-phenylethane
📂 CAS Number — 101-48-4
📘 FEMA Number — 2876
⚖️ Molecular Weight — 166.22 g/mol
📝 Odor Type — Green-floral, aldehydic
📈 Odor Strength — Very strong, powerful (odor detection threshold: 21 ppb in air)
👃🏼 Odor Profile — Fresh green foliage with intense earthy, plant-stem character; hyacinth, lilac, rose, gardenia notes; mushroomy and metallic undertones at high concentrations; subtle honeyed fruity nuances (peach, apricot) in dilution
⚗️ Uses — Green-floral modifier in fine fragrance (hyacinth, lilac, muguet, rose, fougère compositions); stabilizing surrogate for phenylacetaldehyde; flavoring ingredient (≤10 ppm); pharmaceutical and agrochemical intermediate
🧴 Appearance — Colorless to pale yellow clear liquid; density ≈ 1.003-1.004 g/mL at 25°C; refractive index n₂₀/D 1.492-1.495; boiling point 219-221°C at 754 mmHg; flash point 88-90°C (closed cup)
What is Dimethyl Acetaldehyde Phenyl Acetal?
Dimethyl Acetaldehyde Phenyl Acetal, commonly known as PADMA (Phenyl Acetaldehyde DiMethyl Acetal) or by the trade name Viridine®, is a synthetic aromatic acetal belonging to the phenylacetic aldehyde family. With the molecular formula C₁₀H₁₄O₂ and CAS number 101-48-4, this compound is the dimethyl acetal derivative of phenylacetaldehyde (Surburg & Panten, 2006). It is classified as an aliphatic-aromatic compound featuring a phenyl ring connected to an ethane backbone with two methoxy groups at the terminal carbon. The acetal functional group provides superior chemical stability compared to its parent aldehyde, making it particularly valuable in perfumery applications where oxidative stability is critical (Kraft & Swift, 2019).
PADMA is characterized by its intense green-floral odor profile with distinctive hyacinth and lilac notes. The compound serves as a stable alternative to phenylacetaldehyde, offering similar olfactory characteristics without the oxidative instability issues that plague the parent aldehyde. Its presence in fragrance formulations delivers fresh, leafy green notes with earthy undertones that evoke freshly cut flower stems and verdant foliage.
Historical Background
While the exact date of first synthesis remains undocumented in published literature, phenylacetaldehyde dimethyl acetal is believed to have entered commercial perfumery use in the early 1950s as part of the post-war expansion of synthetic fragrance materials (Calkin & Jellinek, 1994). The compound was developed specifically to address the instability challenges associated with phenylacetaldehyde, which readily undergoes oxidation and polymerization, requiring stabilization with antioxidants and dilution with materials such as diethyl phthalate before use (Surburg & Panten, 2006).
Steffen Arctander, in his seminal work Perfume and Flavor Chemicals (1960), documented PADMA as "one of the most widely used 'acetals' in perfumery," noting its versatility across multiple fragrance types. Arctander observed that although always a minor component, the material entered "a multitude of fragrance types where it may lend green notes, earthy notes, floral notes, spicy notes or simply power" (Arctander, 1960, p. 105). His recognition of the material's importance validated its role in the perfumer's palette during the mid-20th century.
The historical significance of PADMA is closely tied to the "green floral" movement of the 1960s and 1970s, when perfumers sought to capture the fresh, natural character of living plants and cut stems rather than just the blooms themselves. PADMA became a foundational building block for hyacinth and lilac reconstructions, often used in conjunction with cis-3-hexenol and other green notes to create realistic flower-stem effects (Curtis & Williams, 2001). Its role as a backbone ingredient in both soliflores and complex fougère compositions established it as an essential tool for achieving naturalistic green-floral accords.
Olfactory Profile
Scent Family: Green-floral, aldehydic
Main Descriptors: The olfactory character of PADMA is dominated by fresh, intense green foliage notes with a distinctive earthy, plant-stem quality. At higher concentrations, the material exhibits strong hyacinth and lilac characteristics with mushroomy, metallic undertones. Upon dilution, honeyed and fruity nuances emerge, particularly reminiscent of peach and apricot. The overall impression is one of freshly cut flower stems with verdant leafy accents and realistic floral undertones (Calkin & Jellinek, 1994).
Intensity: PADMA is characterized by exceptional odor strength with a remarkably low detection threshold of just 21 parts per billion in air (Calkin & Jellinek, 1994). This potency requires judicious use in fragrance formulations, as the material can easily dominate a composition if overdosed. Its powerful diffusive character makes it particularly effective even at low concentrations.
Tenacity: The compound exhibits good substantivity with a longevity of approximately 25-100 hours on a smelling strip, depending on application concentration. This moderate-to-good tenacity positions it primarily as a heart note material, though its diffusive character allows it to contribute to both top and middle note phases of fragrance development.
Volatility: PADMA functions primarily as a middle note ingredient due to its moderate volatility. With a boiling point of 219-221°C at atmospheric pressure, it evaporates more slowly than typical top note materials but faster than base note fixatives. This volatility profile makes it ideal for bridging fresh green top notes with deeper floral heart notes in complex compositions.
Fixative Role: While not a traditional base note fixative, PADMA contributes to the overall stability and longevity of fragrance compositions through its acetal structure. The material helps stabilize more volatile aldehydes and enhances the persistence of green-floral accords. Its ability to blend seamlessly with both volatile and non-volatile materials makes it valuable for creating cohesive fragrance structures.
Applications in Fine Fragrance
PADMA serves multiple critical functions in modern perfumery. Its primary role is as a green-floral modifier in hyacinth, lilac, muguet (lily-of-the-valley), rose, and fougère compositions. The material excels at creating realistic flower-stem and foliage effects that add naturalistic depth to floral accords. Arctander (1960) noted its performance "excellently with Oakmoss and Vetiver, Geranium, Opopanax, etc. in Lilac, Rose, Muguet, Oriental bases, etc. and with the Methylionones in woody-floral-spicy complexes."
In hyacinth reconstructions, PADMA is often combined with phenylacetaldehyde, cis-3-hexenol, and various muguet materials to create the characteristic green-floral freshness of the flower. For lilac accords, it pairs well with terpineol, heliotropin, and various floral alcohols. In rose compositions, it emphasizes earthy-green facets and adds a fresh, dewy quality that evokes morning-cut roses (Curtis & Williams, 2001).
The material is particularly valuable in fougère structures where it contributes green, herbaceous notes that complement lavender, oakmoss, and coumarin. In oriental bases, PADMA can add spicy foliage notes that provide lift and freshness to otherwise heavy, resinous compositions. The material also finds use in gardenia, reseda, and carnation accords where it lends leafy, stem-like character to sweet florals.
Performance in Formula
PADMA exhibits excellent blending behavior in fragrance formulations. The material demonstrates superior oxidative stability compared to phenylacetaldehyde, making it suitable for use in products with extended shelf life requirements. Unlike its parent aldehyde, PADMA does not require special stabilization measures and maintains its olfactory character throughout the product lifecycle (Surburg & Panten, 2006).
In alcoholic fine fragrances, PADMA is typically used at concentrations of 0.5-3% of the fragrance concentrate, though levels may vary depending on the desired effect and the composition type. The material's powerful character requires careful dosing to avoid overwhelming other ingredients. In functional fragrances for household and personal care applications, concentrations are generally lower (0.1-1%) due to the material's intensity and the need for consumer acceptance.
PADMA shows good stability across pH ranges and demonstrates compatibility with both polar and non-polar solvents. It performs well in alcoholic solutions, perfume oils, and various cosmetic bases. The material's acetal structure provides resistance to hydrolysis under normal storage conditions, though extreme pH conditions (very acidic or alkaline) may cause gradual decomposition back to the parent aldehyde.
Synergistic materials include phenylacetaldehyde (for aldehyde reinforcement), phenyl ethyl alcohol (for rose effects), cis-3-hexenol (for green notes), hydroxycitronellal (for muguet accords), and methyl dihydrojasmonate (for floral depth). The material also performs excellently with natural isolates such as geraniol, linalool, and citronellol.
Industrial & Technical Uses
Beyond perfumery, PADMA serves as a FEMA GRAS (Generally Recognized As Safe) flavoring agent under FEMA number 2876, approved for use at concentrations up to 10 ppm in food applications. In flavor formulations, it contributes green, floral notes to fruit flavors, particularly peach and apricot profiles (Adams et al., 2005).
The compound functions as an intermediate in pharmaceutical and agrochemical synthesis, where its acetal protecting group can be selectively removed under controlled acidic conditions to regenerate phenylacetaldehyde. This makes it useful in multi-step organic synthesis where temporary protection of aldehyde functionality is required.
In analytical chemistry, PADMA has been employed in certain detection and quantification methods, though this represents a minor application. The material's well-defined chemical structure and consistent physical properties make it suitable as a reference standard in gas chromatography and mass spectrometry applications.
Regulatory & Safety Overview
IFRA Status: Phenylacetaldehyde dimethyl acetal is not subject to specific restrictions under IFRA (International Fragrance Association) Standards up to and including the 51st Amendment (June 2023). The material is not listed among prohibited or restricted ingredients, indicating no specific concentration limits for use in consumer products (IFRA, 2023). This unrestricted status provides perfumers with flexibility in formulation, though responsible use at appropriate concentrations remains essential.
EU Cosmetics Regulation: PADMA is compliant with EU Cosmetics Regulation (EC) No 1223/2009 and is not listed on Annex II (prohibited substances) or Annex III (restricted substances). The material does not appear on the list of substances subject to mandatory labeling under Regulation (EC) No 1223/2009, as it is not classified as a fragrance allergen requiring declaration at concentrations above threshold levels.
FEMA Status: The compound holds FEMA GRAS status as flavoring ingredient number 2876, with JECFA number 1003. It is listed under 21 CFR 172.515 (U.S. Code of Federal Regulations) as an approved synthetic flavoring substance. The FEMA Expert Panel reviewed safety data and concluded the material is safe for use in food applications at current usage levels (Adams et al., 2005).
Toxicology: According to the RIFM (Research Institute for Fragrance Materials) safety assessment, phenylacetaldehyde dimethyl acetal has been evaluated for dermal sensitization potential, systemic toxicity, and other relevant endpoints. The material demonstrated acceptable safety profiles in standard toxicological testing. Safety evaluation data indicates the material may be harmful if swallowed in concentrated form and is classified as a combustible liquid with a flash point of approximately 88-90°C (Thermo Fisher Scientific, 2025).
Standard safety precautions for handling include use of appropriate personal protective equipment (gloves, eye protection), adequate ventilation, and storage away from heat sources and open flames. The material should be kept in tightly closed containers in a cool, well-ventilated place.
References
Adams, T. B., Cohen, S. M., Doull, J., Feron, V. J., Goodman, J. I., Marnett, L. J., Munro, I. C., Portoghese, P. S., Smith, R. L., Waddell, W. J., & Wagner, B. M. (2005). The FEMA GRAS assessment of phenethyl alcohol, aldehydes, acids, and related acetals and esters used as flavor ingredients. Food and Chemical Toxicology, 43(8), 1179-1206. https://doi.org/10.1016/j.fct.2004.11.016
Arctander, S. (1960). Perfume and flavor chemicals (aroma chemicals) (Vol. 1). Self-published.
Calkin, R. R., & Jellinek, J. S. (1994). Perfumery: Practice and principles. John Wiley & Sons.
Curtis, T., & Williams, D. G. (2001). An introduction to perfumery (2nd ed.). Micelle Press.
IFRA. (2023). IFRA Standards – 51st Amendment. International Fragrance Association. https://ifrafragrance.org/standards-library
Kraft, P., & Swift, K. A. D. (2019). Perspectives on the development of the green-floral note in perfumery. In Perspectives in flavor and fragrance research (pp. 87-134). Verlag Helvetica Chimica Acta.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Wiley-VCH. https://doi.org/10.1002/3527608214
Thermo Fisher Scientific. (2025). Phenylacetaldehyde dimethyl acetal safety data sheet. Retrieved October 2025 from https://www.thermofisher.com