Technical Ingredient Overview
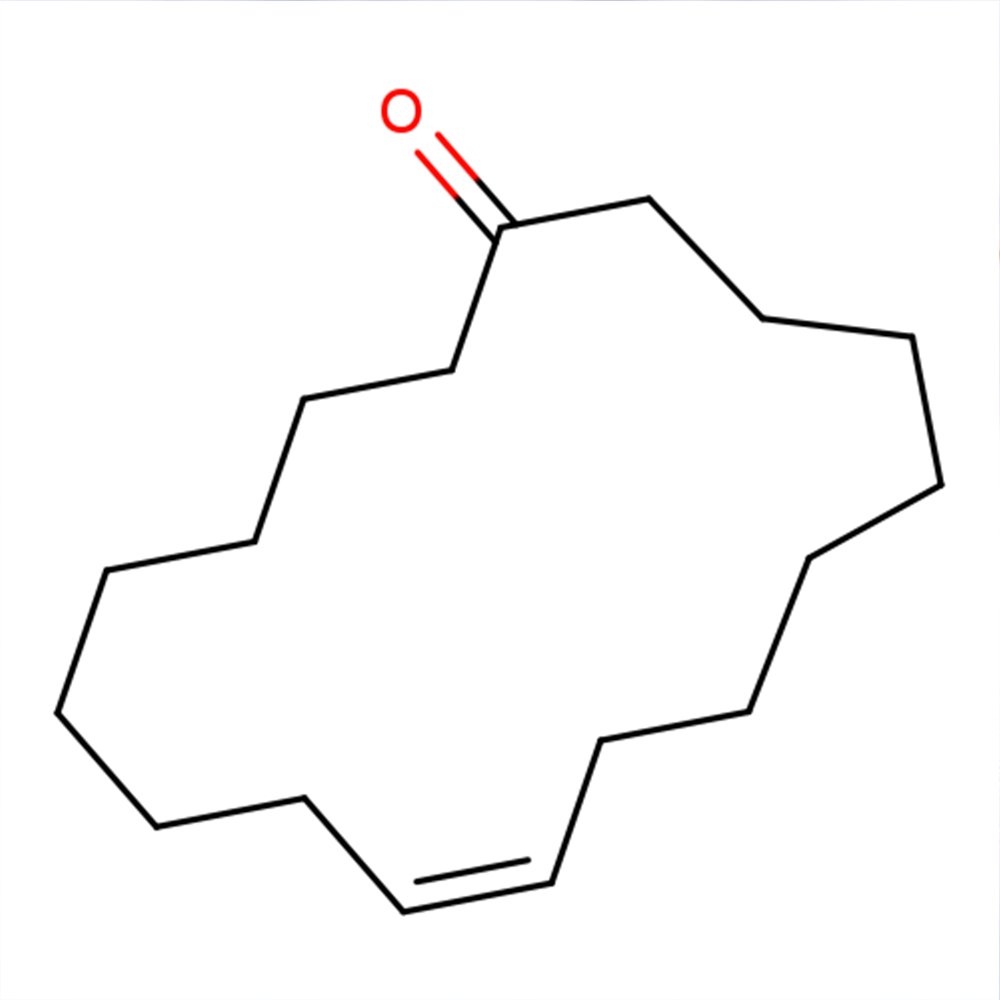
🔎 Chemical Name — (Z)-Cycloheptadec-9-en-1-one
🧪 Synonyms — Civettone, Civetone, (9Z)-Cycloheptadec-9-en-1-one, 1-Cycloheptadecen-10-one, cis-Civetone
📂 CAS Number — 542-46-1
📘 FEMA Number — 2316
⚖️ Molecular Weight — 250.42 g/mol
📝 Odor Type — Musk, Animalic
📈 Odor Strength — Very High; recommended evaluation at 10% dilution or lower
👃🏼 Odor Profile — Intensely warm, musky, animalic, powdery, with delicate sweetness; highly diffusive and exceptionally tenacious; becomes pleasant and radiant at extreme dilutions
⚗️ Uses — Fixative and base note component in fine fragrances; musky accent in floral, oriental, chypre, and aldehydic compositions; civet reconstitution; trace flavoring in specialty applications
🧴 Appearance — Colorless to pale yellow oily liquid; may crystallize at lower temperatures
What is Civetone?
Civetone is a macrocyclic ketone with a 17-membered carbon ring structure and represents one of the most significant odoriferous compounds in classical perfumery. It serves as the principal aromatic constituent responsible for the characteristic musky scent of natural civet oil, historically derived from the perineal gland secretions of the African civet (Civettictis civetta). The compound belongs to an elite category of large-ring musks that revolutionized fragrance chemistry in the early 20th century.
Civetone's molecular architecture features a ketone functional group positioned on a macrocyclic framework containing a cis-configured double bond at the 9-position. This structural complexity, combined with its distinctive olfactory profile, places civetone among the most recognizable and valued materials in perfumery. The natural isomer exhibits the (Z)-configuration, which is essential for its characteristic musky odor; the (E)-isomer demonstrates significantly different olfactory properties.
Modern civetone production relies entirely on synthetic pathways, primarily utilizing precursor chemicals derived from oleic acid found in palm oil and other natural fatty acid sources. This transition from animal-derived extraction to synthetic manufacturing represents a crucial development in ethical perfumery, allowing the industry to preserve traditional olfactory profiles while eliminating animal welfare concerns associated with historical civet farming practices.
Historical Background
The history of civetone is inextricably linked with the broader narrative of musk chemistry and macrocyclic compound synthesis. Natural civet paste, containing civetone as its primary odorant, has been utilized in perfumery for centuries, with documented use dating back to ancient civilizations in Africa, the Middle East, and Asia. The material served dual purposes as both a fragrance ingredient and a fixative, significantly extending the longevity of volatile aromatic compositions.
The structural elucidation of civetone stands as one of the landmark achievements in organic chemistry. Swiss chemist Leopold Ružička, working at the Swiss Federal Institute of Technology (ETH Zurich) in the 1920s and 1930s, successfully determined the molecular structure of both civetone and muscone through degradation studies and synthesis experiments (Ruzicka, 1926). His work challenged prevailing theories of the time, particularly Adolf von Baeyer's strain theory, which suggested that large-ring compounds would be inherently unstable. Ružička demonstrated that macrocyclic ketones with 15- to 17-membered rings not only existed stably but also possessed remarkable olfactory properties.
Ružička's groundbreaking research on macrocyclic compounds earned him the Nobel Prize in Chemistry in 1939, shared with Adolf Butenandt for work on sex hormones. His investigations laid the foundation for synthetic musk chemistry and opened entirely new avenues for fragrance ingredient development. The synthesis of civetone from simpler precursors became feasible in the mid-20th century, though early synthetic routes proved complex and economically challenging.
Commercial synthesis of civetone evolved significantly throughout the latter half of the 20th century. Early methods involved multi-step reactions with limited yields, but advances in catalytic chemistry, particularly olefin metathesis and macrocyclization techniques, dramatically improved efficiency. Contemporary synthetic approaches utilize ring-closing metathesis and other modern organic transformations to produce civetone with high stereochemical purity and commercial viability. The transition to synthetic production became nearly universal by the 1980s, driven by both economic factors and growing ethical concerns about animal-derived ingredients.
Olfactory Profile
Scent Family: Civetone belongs to the musk family, specifically categorized as a macrocyclic musk. It represents the animalic subclass of musks, distinguished from nitro musks, polycyclic musks, and macrocyclic musks of lactone structure.
Main Descriptors: The olfactory profile of civetone exhibits remarkable concentration-dependent characteristics. At undiluted or high concentrations, civetone presents an intensely animalic, fecal, and pungent character that many describe as offensive or overwhelming. However, at appropriate dilutions (typically 10% or lower, often much lower in actual fragrance formulations), the material transforms into a warm, smooth, powdery musk with remarkable radiance and depth. The scent carries subtle sweet undertones, a skin-like quality, and an almost pheromonal warmth that enhances the perceived sensuality of fragrance compositions. Experienced perfumers describe well-diluted civetone as possessing a "lifting" quality that brings roundness and three-dimensionality to floral and oriental accords.
Intensity: Civetone exhibits exceptionally high odor intensity, requiring careful handling and precise dosing in fragrance formulations. Professional evaluation typically occurs at 10% dilution in a suitable carrier such as dipropylene glycol (DPG) or ethanol, though many perfumers prefer even greater dilution for accurate assessment. In finished fragrance concentrates, civetone rarely exceeds 0.1% and often functions effectively at concentrations as low as 0.01% to 0.05%, demonstrating its remarkable potency.
Tenacity: Among natural and synthetic fragrance materials, civetone stands as one of the most tenacious, with exceptional substantivity on both textiles and skin. The compound persists for days or even weeks on perfume blotters and fabrics, contributing significantly to the base note structure and overall longevity of fragrances. This extraordinary fixative power derives from both its molecular weight and structural characteristics, which promote slow evaporation and strong intermolecular interactions with substrates.
Volatility: Civetone functions primarily as a base note material with very low volatility. Its high molecular weight (250.42 g/mol) and macrocyclic structure result in minimal vapor pressure, ensuring slow, sustained release over extended periods. The compound demonstrates practically no top or middle note presence, instead providing foundational support that emerges gradually and persists throughout the entire fragrance development on skin.
Fixative Role: The fixative properties of civetone are legendary in perfumery. The material not only possesses inherent longevity but also demonstrably extends the life of more volatile ingredients in a composition. Civetone interacts with other fragrance components through hydrogen bonding and dispersion forces, effectively reducing their evaporation rates. This fixative effect proves particularly valuable in securing delicate floral notes, citrus oils, and other volatile materials that would otherwise dissipate rapidly. Beyond simple fixation, civetone contributes depth, warmth, and what perfumers describe as "animalic richness" that enhances the overall complexity and perceived quality of fine fragrances.
Applications in Fine Fragrance
Civetone occupies a distinguished position in fine fragrance formulation, where its unique properties serve multiple critical functions. The material proves indispensable in reconstructing classical perfume profiles and creating contemporary fragrances that require exceptional depth, warmth, and longevity.
Role in Fragrance Compositions: In fragrance architecture, civetone functions primarily as a deep base note component and fixative. The material provides structural support to the entire olfactory pyramid, anchoring volatile top and middle notes while contributing its characteristic musky warmth. Civetone adds what perfumers term "animalic richness" or "carnal sensuality" to compositions, creating an olfactory impression of skin, warmth, and intimacy. This quality proves particularly valuable in perfumes designed to enhance rather than mask natural body scent, creating fragrances that feel alive and personal rather than abstract or purely decorative.
Typical Accords and Combinations: Civetone demonstrates remarkable versatility across diverse fragrance families. In floral compositions, particularly those centered on rose, jasmine, orange blossom, or tuberose, civetone adds depth and sensuality while extending longevity. The material pairs exceptionally well with rose materials (phenylethyl alcohol, geraniol, damascones), where it contributes a warm, powdery undertone that enhances the natural impression of rose petals. In jasmine accords, civetone amplifies the natural indolic character while adding musky depth.
Within chypre structures, civetone traditionally appears alongside oakmoss, labdanum, patchouli, and bergamot, contributing animalic warmth that balances the composition's green and woody facets. Oriental fragrances benefit from civetone's ability to harmonize with vanilla, benzoin, amber notes, and warm spices, creating rich, enveloping compositions with exceptional tenacity.
Civetone also appears in aldehydic fragrances, where its warm, skin-like quality provides grounding for the bright, sparkling effects of aliphatic aldehydes. The contrast between civetone's smooth muskiness and aldehydes' sharp, soapy-waxy character creates sophisticated tension that characterizes many classical perfumes.
Pairing Behavior with Other Materials: Civetone exhibits synergistic relationships with numerous fragrance ingredients. The material blends harmoniously with other musk compounds (galaxolide, muscone, ambrettolide), creating complex musky bases with enhanced depth and character. When combined with woody materials such as cedarwood derivatives, sandalwood components, or synthetic woods (Iso E Super, Javanol), civetone adds warmth and sensuality while the woods contribute dry, refined elegance.
Civetone's interaction with animalic materials deserves particular attention. When used with castoreum reconstitutions, leather accords, or indolic components (indole, skatole, methylanthranilate), civetone provides smooth integration and modulation, preventing excessive harshness while maintaining animalic character. The material also demonstrates excellent compatibility with ambery materials (Ambrox, ambroxan, labdanum), creating warm, skin-scent impressions of remarkable sophistication.
Performance in Different Fragrance Types: Civetone's performance varies across fragrance applications. In fine fragrance (eau de parfum and parfum concentrations), the material functions optimally, providing its full range of effects including fixation, depth, and sensuality. In eau de toilette concentrations, civetone remains effective though its presence may be somewhat less pronounced due to lower overall perfume oil content.
In functional fragrances (body care, household products), civetone use requires careful consideration due to its intensity and animalic character. While the material can provide exceptional longevity and distinctiveness in premium body care products, its powerful odor may prove too assertive for mass-market applications where consumer preferences favor cleaner, lighter profiles. In fine fragrance candles and luxury home fragrance, civetone contributes warmth and complexity when used judiciously.
Performance in Formula
Behavior in Blends and Mixtures: Civetone demonstrates excellent solubility in ethanol, perfumers alcohol, and other common fragrance carriers, though it may require gentle warming for complete dissolution when working with concentrated material or at lower temperatures. The compound exhibits outstanding stability in alcoholic solutions, maintaining its olfactory profile for extended periods without significant degradation.
In oil-based formulations, civetone dissolves readily and shows excellent compatibility with both natural essential oils and synthetic aroma chemicals. The material demonstrates no tendency toward crystallization in properly formulated blends, though pure or highly concentrated civetone may solidify at ambient temperatures below approximately 15-18°C.
Diffusion Characteristics: Despite its low volatility and base note classification, civetone possesses surprising diffusive power. The material projects remarkably well from skin and textiles, creating a perceptible musky aura that extends beyond the immediate application site. This diffusion occurs through a combination of active evaporation (albeit slow) and what perfumers describe as "radiance" – the ability to make other materials more perceptible and to create an impression of lift and projection.
The diffusion profile of civetone follows a distinctive pattern: initially subtle or even imperceptible in the early stages of fragrance development, the material gradually emerges as more volatile components dissipate, revealing its full character in the base and providing sustained presence over many hours or days. This delayed emergence creates a sense of evolving complexity in well-structured fragrances.
Impact on Overall Composition: Civetone exerts profound influence on fragrance character far beyond what its low concentration might suggest. Even at levels of 0.01% to 0.05% in the concentrate, civetone significantly impacts the overall impression, adding warmth, depth, and complexity. The material functions as what perfumers call a "modifier" – an ingredient that transforms the character of other materials and the composition as a whole rather than contributing a dominant recognizable note.
Civetone's presence enhances the perceived quality and sophistication of fragrances, creating an impression of richness and fullness. The material adds what is sometimes described as a "three-dimensional" quality, making fragrances feel more complex, natural, and alive. This effect proves particularly valuable in compositions that might otherwise seem flat, linear, or overly synthetic.
Fixative Strength and Properties: The fixative mechanism of civetone operates through multiple pathways. The compound's high molecular weight and low vapor pressure provide inherent longevity, while its ability to form weak intermolecular associations with other fragrance materials reduces their evaporation rates. Civetone appears to create a subtle matrix effect in fragrance formulations, potentially slowing diffusion of more volatile components through physical interaction.
Quantitative studies suggest that civetone can extend the half-life of certain volatile materials by 20-50% or more, though exact figures depend on the specific materials and formulation context. This effect proves most pronounced with moderately volatile materials (middle notes) rather than highly volatile top notes, which evaporate too rapidly for fixation, or other base notes, which already possess sufficient longevity.
Compatibility with Other Materials: Civetone demonstrates broad compatibility across the fragrance palette with few restrictions. The material blends harmoniously with essentially all natural essential oils, absolutes, and synthetic aroma chemicals. No significant incompatibilities, discolorations, or instabilities have been reported in standard fragrance applications.
The material shows particular affinity for indolic components, with which it forms smooth, integrated animalic bases rather than creating harsh combinations. With woody materials, civetone provides warming effects without muddiness. In floral contexts, the material adds depth without overshadowing delicate nuances when properly dosed.
Industrial & Technical Uses
Non-Fragrance Applications: Beyond perfumery, civetone finds limited but notable applications in other industries. In flavor science, the compound appears in FEMA's GRAS (Generally Recognized As Safe) database as FEMA 2316, with approved use in food flavoring at extremely low concentrations (maximum 0.0500 ppm). In this context, civetone contributes to complex flavor profiles in specialty applications, particularly in recreating certain fruit flavors (especially exotic fruits), tobacco flavoring, and in some savory applications where minute amounts of musky, animalic notes add depth and complexity.
The compound has found unexpected application in wildlife biology and conservation research. Field biologists studying large cats, particularly jaguars, have employed commercial fragrances containing civetone as attractants for camera traps. Jaguars demonstrate marked interest in certain perfumes, likely due to the presence of musky, animalic compounds that mimic territorial marking pheromones. This application has proven valuable for population surveys and behavioral studies of elusive wild cats.
Industrial Processes and Functions: In the fragrance manufacturing industry, civetone serves as an important reference material for quality control, olfactory training, and analytical work. The compound appears in many professional perfumery educational programs as an essential material for understanding musk chemistry, fixative principles, and animalic notes.
From a chemical synthesis perspective, civetone represents an important target molecule for demonstrating new synthetic methodologies, particularly in macrocyclization chemistry. The compound frequently appears in academic and industrial research as a test case for novel ring-closing reactions, olefin metathesis catalysts, and other synthetic transformations relevant to complex organic synthesis.
Technical Specifications and Requirements: Commercial civetone typically meets specifications of ≥95% purity as determined by gas chromatography, with the (Z)-isomer predominating. The material should be stored in tightly sealed containers, protected from light, air, and elevated temperatures to prevent oxidation and isomerization. Optimal storage conditions include temperatures between 15-25°C in amber glass containers with minimal headspace.
For perfumery applications, civetone should be free from off-odors, discoloration, and visible particles. The material should dissolve completely in ethanol at room temperature (with gentle warming if necessary), producing a clear to slightly hazy solution depending on concentration and temperature. Refractive index, specific gravity, and other physical constants should fall within established ranges for quality verification.
Regulatory & Safety Overview
IFRA Status: Civetone is not restricted under the current IFRA Standards (51st Amendment, 2024). The material does not appear on IFRA's list of prohibited or restricted ingredients, meaning it may be used without specific concentration limits in fragrance applications. However, as with all potent materials, formulators must consider appropriate dosing to ensure consumer safety and product acceptability. The International Fragrance Association continues to monitor all fragrance ingredients through its ongoing safety assessment program, and formulators should consult the most current IFRA Standards for any updates to regulatory status. For current information, consult: https://ifrafragrance.org/priorities/ingredients/ifra-standard
EU Cosmetics Regulation: Civetone is not listed among the 26 declarable allergens specified in Annex III of EU Regulation (EC) No 1223/2009. This means the ingredient does not require specific declaration on finished product labels when used in cosmetic and personal care products sold in the European Union, though it must be included in the overall ingredients list using INCI nomenclature if present above threshold concentrations.
The material is permitted for use in cosmetics and personal care products in the EU, provided that finished products meet safety requirements and good manufacturing practices. As civetone is not restricted under the Cosmetics Regulation, no specific concentration limits apply, though responsible formulation practices dictate appropriate use levels based on safety assessments and consumer acceptability.
FEMA Status: Civetone is approved for use as a flavoring substance under FEMA (Flavor and Extract Manufacturers Association) number 2316. The compound holds GRAS (Generally Recognized As Safe) status for its intended use in food flavoring, with a recommended maximum use level of 0.0500 parts per million (ppm) in finished food products. This classification reflects evaluation by expert panels concluding that civetone poses no safety concerns when used in minute quantities typical of flavoring applications. For verification, consult the FEMA GRAS database at: https://www.femaflavor.org/fema-gras
Environmental and Sustainability Considerations
Modern civetone production from plant-derived precursors (primarily oleic acid from palm oil or other vegetable oils) represents a significant improvement in sustainability compared to animal-derived sources. However, the use of palm oil-derived feedstocks raises concerns about deforestation, habitat destruction, and biodiversity loss associated with palm oil cultivation. Responsible manufacturers increasingly source from certified sustainable palm oil programs (RSPO certification) or utilize alternative oleic acid sources including olive oil derivatives, sunflower oil, or synthetic routes not dependent on potentially problematic agricultural feedstocks.
From a biodegradation perspective, civetone demonstrates moderate biodegradability under aerobic conditions, though the macrocyclic structure degrades more slowly than simpler aliphatic or aromatic compounds. The material shows low bioaccumulation potential despite its lipophilicity, with predicted bioconcentration factors suggesting limited environmental persistence in aquatic organisms. Nonetheless, the GHS classification as toxic to aquatic life with long-lasting effects necessitates proper waste handling and disposal procedures to minimize environmental release.
The transition from animal-derived civet to synthetic civetone represents one of perfumery's most significant ethical advances. Historical civet production involved maintaining civet cats in captivity and periodically extracting glandular secretions – a practice raising substantial animal welfare concerns. The availability of synthetic civetone has largely eliminated demand for animal-derived material, though small-scale traditional production may persist in some regions. Modern perfumers can achieve identical or superior olfactory effects using synthetic material while avoiding ethical concerns associated with animal ingredient sourcing.
Sources:
Arctander, S. (1969). Perfume and flavor materials of natural origin. Allured Publishing Corporation.
Arctander, S. (1994). Perfume and flavor chemicals (aroma chemicals). Allured Publishing Corporation.
European Chemicals Agency (ECHA). (n.d.). Substance information: Civetone [CAS 542-46-1]. Retrieved from https://echa.europa.eu
Flavor and Extract Manufacturers Association (FEMA). (2024). FEMA GRAS database: FEMA No. 2316. Retrieved from https://www.femaflavor.org/fema-gras
International Fragrance Association (IFRA). (2024). IFRA Standards (51st Amendment). Retrieved from https://ifrafragrance.org/priorities/ingredients/ifra-standard
Kraft, P., & Bajgrowicz, J. A. (2005). Odorants. In Ullmann's encyclopedia of industrial chemistry. Wiley-VCH.
Michigan State University Department of Chemistry. (2024). Leopold Ruzicka. Faculty Research Portraits. Retrieved from https://www.chemistry.msu.edu/faculty-research/portraits/ruzicka-leopold.aspx
National Center for Biotechnology Information. (2024). PubChem Compound Summary for CID 5315941, Civetone. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Civetone
Ohloff, G., Pickenhagen, W., & Kraft, P. (2012). Scent and chemistry: The molecular world of odors. Wiley-VCH.
Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Official Journal of the European Union, L 342/59.
Research Institute for Fragrance Materials (RIFM). (2024). Safety assessment of fragrance materials. The RIFM Database. Retrieved from https://www.rifm.org
Rossiter, K. J. (1996). Structure-odor relationships. Chemical Reviews, 96(8), 3201-3240.
Ruzicka, L. (1926). The life and work in Geneva from 1929 to 1957. Proceedings of the Chemical Society, January 1960, 341-360.
Sell, C. S. (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Royal Society of Chemistry.
Sell, C. S. (2014). Chemistry and the sense of smell. John Wiley & Sons.
Surburg, H., & Panten, J. (2016). Common fragrance and flavor materials: Preparation, properties and uses (6th ed.). Wiley-VCH.
United Nations. (2021). Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (Rev. 9). United Nations Publications.
Zviely, M. (2012). Revisit to (Z)-civetone synthesis. Natural Product Communications, 7(7), 853-858. https://doi.org/10.1177/1934578X1200700728