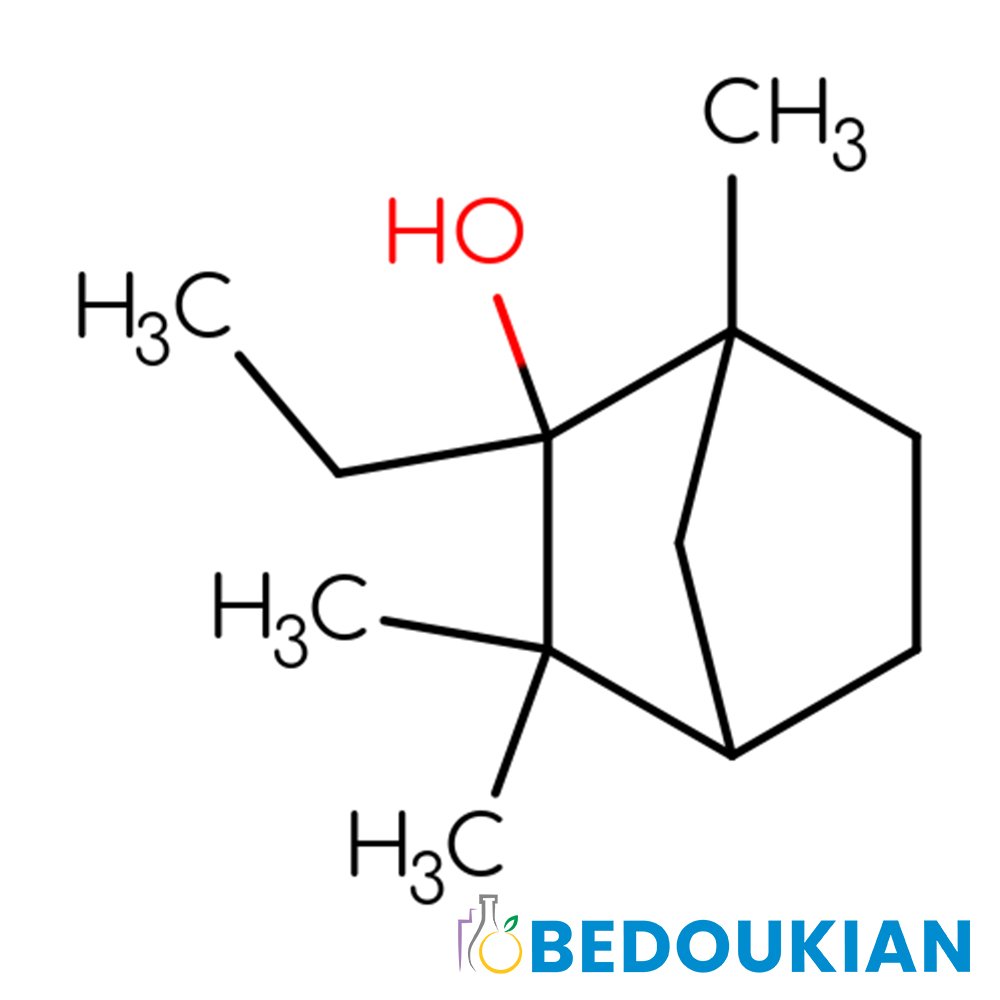
Technical Ingredient Overview
🔎 Chemical Name — endo-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
🧪 Synonyms — Borneo camphor, d-Borneol, l-Borneol, Bornyl alcohol, 2-Camphanol, Baros camphor, Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, endo-Borneol, 2-Hydroxycamphane
📂 CAS Number — 507-70-0
📘 FEMA Number — 2157
⚖️ Molecular Weight — 154.25 g/mol
📝 Odor Type — Balsamic, camphoraceous
📈 Odor Strength — Medium (moderate intensity)
👃🏼 Odor Profile — Dry camphoraceous with pine-like and woody undertones; slightly peppery and minty character; cleaner and less pungent than camphor; exhibits subtle balsamic nuances; differs between enantiomers with l-borneol presenting a cleaner, less sharp profile
⚗️ Uses — Perfumery (incense accords, lavender compositions, fougère fragrances, pine notes, aromatic structures); flavor applications (spice and nut profiles); essential oil reconstitution (rosemary, lavandin); traditional medicine; natural insect repellent
🧴 Appearance — Colorless to white crystalline solid or amorphous powder; transparent crystals when pure
What is Borneol?
Borneol is a bicyclic monoterpenoid alcohol that belongs to the broader class of terpene compounds derived from the isoprene biosynthetic pathway. Chemically classified as an endo-isomer of the bicyclo[2.2.1]heptane ring system, borneol contains a hydroxyl (-OH) functional group positioned at the C-2 carbon in the endo configuration, distinguishing it from its structural isomer isoborneol, which bears the hydroxyl group in the exo position.
The compound exists as two optical isomers: d-(+)-borneol (dextrorotatory) and l-(−)-borneol (levorotatory), both of which occur naturally in various plant species including Dryobalanops aromatica (Borneo camphor tree), rosemary (Rosmarinus officinalis), lavender species, and numerous coniferous plants. However, commercial borneol used in fragrance and flavor industries is predominantly synthetic, produced either through chemical reduction of camphor or via semi-synthetic routes starting from α-pinene.
Industrial synthesis methods include:
Meerwein-Ponndorf-Verley (MPV) reduction of camphor using aluminum isopropoxide
Sodium borohydride reduction of camphor in alcoholic solvents
Catalytic hydrogenation of camphor over metal catalysts
Semi-synthetic conversion from α-pinene through multiple chemical steps
Synthetic borneol is typically obtained as a racemic mixture (dl-borneol) or as partially enriched enantiomeric forms depending on the production method and starting materials. The racemate exhibits intermediate olfactory characteristics between the pure enantiomers.
Historical Background
The history of borneol is intimately connected with traditional Asian commerce and medicine, predating modern organic chemistry by centuries. Natural borneol, known as "Borneo camphor" or "dragon's brain camphor" (long nao xiang in Chinese), was historically obtained from Dryobalanops aromatica, a tree native to Borneo and Sumatra. This precious crystalline material was found in cracks and cavities within the trunk of mature trees and was traded throughout Asia as early as the 5th century CE for its medicinal properties and use in religious ceremonies.
The first chemical isolation and characterization of borneol as a distinct compound separate from common camphor (from Cinnamomum camphora) occurred in the mid-19th century. French chemist Élie-Abel Carrière and others working with natural products from Southeast Asian sources identified its unique chemical structure and properties. The systematic chemical synthesis of borneol was achieved in the late 19th and early 20th centuries as organic chemistry advanced.
The German chemist Otto Wallach, who received the Nobel Prize in Chemistry in 1910 for his work on alicyclic compounds, contributed significantly to understanding the stereochemistry and structural relationships between borneol, isoborneol, and camphor. His work established the foundation for modern terpene chemistry and enabled synthetic production methods.
Commercial synthetic production of borneol began in the early 20th century, primarily through reduction of camphor, which was more readily available than natural Borneo camphor. This development made borneol accessible for industrial fragrance and flavor applications. The Meerwein-Ponndorf-Verley reduction method, developed in the 1920s, provided a reliable synthetic route that remains in use today.
Key milestones in borneol's development:
5th-10th centuries: Trade in natural Borneo camphor across Asia
1850s-1860s: Chemical isolation and initial characterization
1890s-1910s: Structural elucidation and stereochemical studies by Wallach and contemporaries
1920s: Development of MPV reduction for commercial synthesis
1960s-1970s: Recognition as FEMA GRAS flavor ingredient (FEMA 2157)
1980s-present: Integration into modern perfumery and continued use in essential oil reconstitution
Olfactory Profile
Scent Family: Borneol belongs to the camphoraceous-woody olfactory family, with secondary classification within the balsamic and herbal categories.
Main Descriptors:
Primary: Dry camphoraceous, pine-like, woody
Secondary: Balsamic, slightly peppery, minty-cool
Tertiary: Herbal-green, subtle resinous, faintly medicinal
The olfactory character of borneol is notably drier and less penetrating than camphor itself, presenting a more refined and less aggressive camphoraceous note. The pine-like quality is prominent, accompanied by a woody dryness that distinguishes it from the more aggressive bite of camphor or the sweeter profile of isoborneol. A subtle peppery-minty coolness emerges in the dry-down, contributing to its utility in aromatic and fougère compositions.
Enantiomeric differences are detectable to trained noses: l-(−)-borneol presents a cleaner, less harsh camphoraceous character with more pronounced balsamic undertones, while d-(+)-borneol exhibits slightly sharper, more pronounced woody-pine aspects. Commercial racemic borneol presents intermediate characteristics.
Intensity: Medium odor strength at standard use levels (0.5-3% in fragrance concentrate). The compound exhibits good diffusion without overwhelming other fragrance components, making it suitable as a secondary modifier rather than a dominant note.
Tenacity: Moderate tenacity with a half-life in the medium range on blotter strips. While classified as a top-to-heart note, borneol demonstrates better persistence than many monoterpene alcohols due to its bicyclic structure, which reduces volatility compared to acyclic or monocyclic analogs. In formulations, it can persist for 4-8 hours on skin depending on concentration and surrounding materials.
Volatility: Moderate volatility positioning borneol in the top-to-heart transition zone. Its relatively low vapor pressure compared to camphor (due to hydrogen bonding capability) results in a more gradual evaporation profile. The compound exhibits an evaporation rate approximately 60-70% that of camphor, placing it functionally between typical top notes and true heart materials.
Fixative Role: Borneol demonstrates mild fixative properties, contributing to fragrance stability through hydrogen bonding interactions with other oxygen-containing fragrance materials. While not a strong fixative like heavier molecules (e.g., musks, resins), it can improve the staying power of lighter terpenic and citrus components. Its bicyclic structure provides some substantivity to formulations, helping to moderate the evaporation of more volatile materials in aromatic-citrus and fougère compositions.
Applications in Fine Fragrance
Borneol occupies a specialized niche in perfumery as a secondary modifying agent rather than a featured keynote. Its primary value lies in its ability to contribute structural complexity and naturalistic character to various fragrance categories.
Role in Fragrance Compositions:
Aromatic Fougères: Provides authentic herbal-woody backbone supporting lavender, oakmoss, and coumarin structures
Incense and Oriental Accords: Contributes dry camphoraceous-woody notes that enhance frankincense, myrrh, and cistus compositions
Pine and Conifer Notes: Essential component in reconstituted pine needle, spruce, and fir balsam accords
Lavender Compositions: Adds naturalness and complexity to both lavandin and true lavender reconstructions
Cologne and Aromatic Citrus: Provides herbal-woody bridge between citrus top notes and woody-amber bases
Typical Accords and Combinations:
With olibanum/frankincense: Creates authentic incense effects with enhanced diffusion
With lavender oils: Reinforces natural camphoraceous aspects while adding dryness
With citrus terpenes (limonene, pinene): Provides aromatic lift and improved tenacity
With rosemary and sage: Enhances herbal authenticity in aromatic structures
With cedarwood and vetiver: Contributes to naturalistic woody-earthy compositions
Pairing Behavior: Borneol demonstrates excellent blending characteristics with:
Terpenic materials (α-pinene, β-pinene, limonene)
Lavender oils and synthetic lavender components
Camphoraceous materials (camphor, eucalyptol)
Woody notes (cedarwood derivatives, vetiver)
Herbaceous absolutes (clary sage, rosemary)
Light balsamic materials (Peru balsam, benzoin)
Performance in Different Fragrance Types:
Eau de Cologne (2-5%): Functions well at lower concentrations (0.5-1.5%), providing herbal lift without overwhelming citrus freshness
Eau de Toilette (5-15%): Optimal performance range (1-3%), contributing to aromatic heart development
Eau de Parfum (15-20%): Can be used at similar levels but may appear sharper due to higher alcohol concentration
Functional Fragrances: Particularly effective in room sprays, laundry fragrances, and cleaning products (up to 3%)
Recommended Use Levels: 0.5-3% in fragrance concentrate, with typical applications at 1-2% for optimal balance.
Performance in Formula
Behavior in Blends and Mixtures: Borneol exhibits excellent solubility in ethanol and other fragrance solvents, though care must be taken with temperature to ensure complete dissolution, particularly at higher concentrations. The compound demonstrates good stability in both alcoholic and oil-based formulations, with no significant hydrolysis or oxidation under normal storage conditions. However, prolonged exposure to strong bases may lead to dehydration reactions.
In complex mixtures, borneol acts as a "bridge" material, helping to harmonize volatile terpenic top notes with more substantive heart materials. Its moderate polarity (due to the hydroxyl group) allows it to interact favorably with both hydrophobic and hydrophilic fragrance components, contributing to overall blend cohesion.
Diffusion Characteristics: Borneol demonstrates good radial diffusion with a relatively even evaporation profile. Unlike some highly volatile materials that create an immediate impact followed by rapid decline, borneol provides sustained diffusion over the first 30-90 minutes of wear. This makes it valuable for maintaining aromatic character during the critical early-to-middle phase of fragrance development.
The compound's diffusion is enhanced when combined with materials having similar volatility profiles (e.g., linalool, lavandulyl acetate, eucalyptol), creating a cohesive aromatic cloud. Conversely, when paired with very heavy materials, borneol helps lift and project the composition during initial application.
Impact on Overall Composition:
Aromatic Character: Adds naturalistic herbal-woody dimension to synthetic structures
Freshness: Contributes cooling, clean aspects without overt mintiness
Complexity: Provides structural depth to simple citrus or lavender bases
Balance: Helps moderate overly sweet or powdery accords
Naturalness: Improves perceived authenticity of essential oil reconstructions
Fixative Strength and Properties: While borneol is not classified as a true fixative in the traditional sense, it does contribute to formula stability through several mechanisms:
Hydrogen bonding with aldehydes, ketones, and esters reduces their volatility
Bicyclic structure provides some substantivity
Moderate evaporation rate helps anchor lighter terpenic materials
Synergistic effects with other fixatives (e.g., galaxolide, iso E super)
The fixative contribution is most noticeable in citrus-aromatic and fougère compositions, where borneol can extend the life of bergamot, lavender, and herbaceous notes by 15-25%.
Compatibility with Other Materials: Borneol demonstrates broad compatibility but specific considerations include:
Excellent compatibility: Lavender oils, rosemary, pine derivatives, citrus terpenes, light musks, cedrol, vetiver
Good compatibility: Coumarin, oakmoss, sage, eucalyptol, camphorous materials
Moderate compatibility: Vanilla, heliotropin (may create unusual contrasts)
Poor compatibility: Heavy amber bases (may appear discordant), pronounced fruity esters (can create off-notes)
Industrial & Technical Uses
Beyond perfumery, borneol finds application across several industrial sectors:
Flavor Industry: As a FEMA GRAS-approved flavoring substance (FEMA 2157), borneol is used in small quantities to impart woody, slightly minty, and spicy notes to food products. Applications include:
Nut flavor profiles (walnut, almond)
Spice blends and seasoning formulations
Herbal flavor systems (rosemary, sage reconstructions)
Beverage flavoring (particularly in traditional and herbal drinks)
Typical use levels in flavors are significantly lower than in fragrances (0.01-0.5 ppm in finished food products) due to its relatively strong taste impact.
Essential Oil Reconstitution: Borneol serves as a key component in the reconstitution and standardization of natural essential oils, particularly:
Rosemary oil (Rosmarinus officinalis)
Lavandin oil (Lavandula × intermedia)
Valerian oil (Valeriana officinalis)
Sage oils (various Salvia species)
The addition of synthetic borneol can adjust the natural variability of essential oils to meet specifications or extend limited natural supplies.
Traditional Medicine and Pharmaceuticals: Borneol has a long history in traditional Chinese medicine (long nao xiang) and Ayurvedic medicine, used for:
Topical analgesic formulations
Anti-inflammatory preparations
Cardiovascular support formulations (traditional use)
Aromatic stimulant preparations
In modern pharmaceutical applications, borneol serves as:
Permeation enhancer for transdermal drug delivery
Component in topical pain relief products
Ingredient in some respiratory relief preparations
Chemical Industry:
Camphor Synthesis: Borneol is readily oxidized to camphor, allowing its use as a camphor precursor in certain synthetic routes
Resin Formulations: Employed in specialty aromatic resins for incense manufacturing
Natural Insect Repellents: Acts as a mild repellent in natural insecticide formulations
Analytical Chemistry: Used as a reference standard in GC-MS and other analytical methods for terpene analysis
Other Applications:
Component in aromatic woods and papers for traditional religious and cultural practices
Ingredient in some traditional ink formulations
Natural preservative in certain traditional food preservation methods (Asia)
Research reagent in studies of terpene biosynthesis and metabolism
Regulatory & Safety Overview
IFRA Status: Borneol is not restricted under the current IFRA Standards (51st Amendment, 2024). No specific use level limitations apply, and the material may be used in fragrance products without IFRA-mandated restrictions. However, formulators should still observe general good manufacturing practices and adhere to finished product regulations in target markets.
IFRA documentation: https://ifrafragrance.org/standards-library
EU Cosmetics Regulation: Borneol is not listed among the 26 declarable fragrance allergens in Annex III of EU Regulation 1223/2009 on cosmetic products. Therefore, it does not require specific declaration on cosmetic product labels beyond general "parfum/fragrance" listing. The substance is considered safe for use in cosmetic products when used according to good manufacturing practices.
FEMA Status: Borneol holds FEMA GRAS (Generally Recognized As Safe) status with the designation FEMA 2157. It is approved for use as a flavoring agent in food products under conditions of intended use. The FEMA Expert Panel has evaluated the safety of borneol for flavor applications based on available toxicological data and historical use patterns.
Sources:
Arctander, S. (1969). Perfume and flavor materials of natural origin. Allured Publishing Corporation.
Arctander, S. (1994). Perfume and flavor chemicals (aroma chemicals). Allured Publishing Corporation.
Bauer, K., Garbe, D., & Surburg, H. (2001). Common fragrance and flavor materials: Preparation, properties and uses(4th ed.). Wiley-VCH.
European Chemicals Agency (ECHA). (n.d.). Borneol - Substance information. Retrieved from https://echa.europa.eu
FEMA (Flavor and Extract Manufacturers Association). (n.d.). Borneol - FEMA 2157. FEMA GRAS Database. Retrieved from https://www.femaflavor.org/flavor-library/borneol
IFRA (International Fragrance Association). (2024). IFRA Standards (51st Amendment). Retrieved from https://ifrafragrance.org/standards-library
Kraft, P., & Swift, K. A. D. (2000). Perspectives on the development of new odorants. Chemistry and Biodiversity, 8(11), 1-24.
National Center for Biotechnology Information. (n.d.). PubChem Compound Summary for CID 64685, Borneol. Retrieved from https://pubchem.ncbi.nlm.nih.gov/compound/Borneol
Sell, C. S. (2006). The chemistry of fragrances: From perfumer to consumer (2nd ed.). Royal Society of Chemistry.
Sell, C. S. (2010). Chemistry and the sense of smell. John Wiley & Sons.
Surburg, H., & Panten, J. (2006). Common fragrance and flavor materials: Preparation, properties and uses (5th ed.). Wiley-VCH.