Ifra 51st Amendment
Ifra 51st Amendment
Premise
The here presented article has the purpose of listing and explaining some of the changes that have been introduced in the 51st Amendment, notified by IFRA on the 30th of June, 2023. In no way, shape, or form does this article have to be seen as a substitute for the direct consultation of the amendment itself. Thus, Scentspiracy encourages a proper and personal exploration of the norm that will be available at the following link on the IFRA website.
Appendix
Due to the extreme specificity of the text, a brief appendix, listing and explaining certain words or categories that will later be mentioned is rendered essential in order to grant a proper and clear consultation of the article.
MAC: Maximum Acceptable Concentrations
RMTF: Risk Management Task Force
QRA2: Quantitative Risk Assessments
TTC: Threshold of Toxicological Concern
Category 5B: Leave on products applied to the face and body using the hands (palms)
Category 6: Products with lip and oral exposure
Category 10 A: Household care products with mostly hand contact
Category 11B: Products with intended skin contact but minimal transfer of fragrance to skin from inert substrate
Category 12: Products not intended for direct skin contact, minimal or insignificant transfer to skin
What are the IFRA Standards?
The International Fragrance Association, also known as IFRA, was founded in 1973 with the intent of representing the interests of the fragrance industry worldwide. In order to see their goal come to fruition, throughout the years IFRA has released a series of guidelines, usually integrated with amendments, providing a comprehensive approach to products such as perfumes, cosmetics, and household goods.
These guidelines and amendments represent IFRA’s concrete strive towards the creation of a more sustainable, safe, and transparent environment for both perfumers and consumers alike. Both the guidelines and the amendments naturally contribute to the formation of the so-called IFRA Standards, an ever-changing set of criteria that aims at the restriction, prohibition, limitation, or changes in the categorization of certain ingredients or products that may be recognized as hazardous for the environment and human interaction. Moreover, IFRA has also added an Annex inside which discusses and compiles contributions to the IFRA Standards derived from other sources, combining information from natural contributions formerly known as Annex I, as well as Schiff bases, previously known as Annex II.
51st Amendment Implementation Timeline
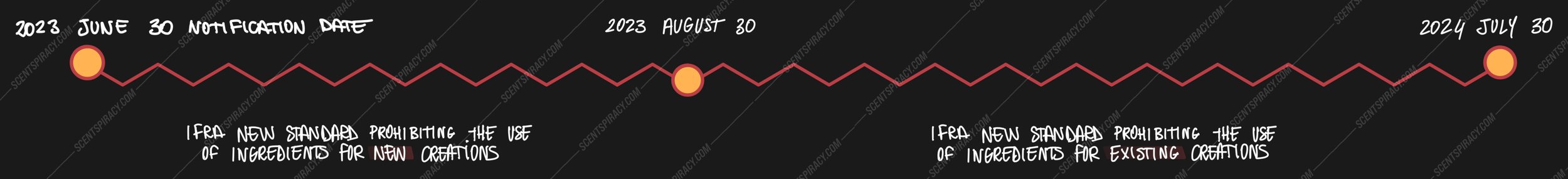
The 51st Amendment to the IFRA Standards was published on the 3rd of July, 2023, and it was notified with a letter dated June 30th. Its publication came with a comprehensive timeline of the expected implementation of said norms, providing different timespans for new restrictions and prohibitions.
Thus, IFRA has divided the implementation timeline into two categories: one for new ingredient restrictions and specifications, and the other for prohibited ingredients. Moreover, for each of the above-mentioned categories, two different implementation timelines have been indicated, dividing both the Restriction and the Prohibition sections in New Creations and Existing Creations.
With regard to the Restriction or Specification section, all IFRA members will necessitate an IFRA Certificate of Conformity in compliance with the 51st Amendment by the following dates:
New Creations: March 30th, 2024 (9 months after the notification date)
Existing Creations: October 30th, 2025 (28 months after the notification date)
On the other hand, the dates of compliance regarding the Prohibition Standard should be implemented by the following dates:
New Creations: August 30th, 2023 (2 months after the notification date)
Existing Creations: July 30th, 2024 (13 months after the notification date)
It is of the utmost importance to stress that the Prohibition timeline only applies to formulas, and not to the commercialized finished product.
Section 2 Summary: Relevant Changes to the Guidance for the Use of the IFRA Standards
Section 2 of the 51st Amendment IFRA aims to clarify certain aspects which emerged from feedback received on the Guidance for the use of the IFRA Standards, dividing this portion of the text into nine sub-sections.
Sub-Section 2.1 - Clarification about paper products:
Necessity of clearer wording regarding paper products when calculating fragrance ingredient concentrations in finished products. IFRA specifies that the above-mentioned products affected by said clarifications are: Baby diapers, Feminine hygiene conventional pads, liners, Interlabial pads, Tampons, Incontinence pants/pads, Cleaning wipes, Baby wipes, Dryer sheets, and Wet toilet paper.
Sub-Section 2.2 - Clarification about the categorization of fabric softener sheets, dryer sheets, and dry-cleaning kits:
IFRA concentrated on dispelling some nebulous categorizations which interested the above-mentioned products. Both Fabric Softener Sheets (previously catalogued in Category 10 A) and Dryer Sheets are now new entries in Category 12. When it comes to Dry Cleaning Kits, IFRA decided to apply further discrimination between two types:
The first typology is placed in the dryer and hence, having limited contact with the skin, should be qualified as Category 12;
The second type which involves active manual application, such as rubbing on the clothes, should be placed in Category 10A instead.
Sub-Section 2.3 - Categorization of Pillow Sprays:
Clarifications were asked about the presence of pillow spray in Category 11B. Although it was suggested to move the product to Category 5B or 10A, IFRA confirmed that the product would remain in its original category, stating that said classification derived largely from skin sensitization concerns and potential transfer of the product from the pillow to the skin.
Sub-Section 2.4 - Categorization of Reed Diffusers:
Some impactful changes of the MAC for Category 10A of two revised Standards of Geraniol (CAS N. 106-24-1) and Hydroxycitronellal (CAS N. 107-75-5), which may potentially impact reed diffusers.
Sub-Section 2.5 - Handling of Traces:
When questioned about the concept of responsibility highlighted in the current Guidance, the RMTF found the current text adequate in describing the roles and responsibilities of exchanging information along the supply chain for responsible management of traces in finished consumer products.
Sub-Section 2.6 - Phototoxicity Considerations for Products in Category 6:
In regard to some clarifications asked about the presence of phototoxicity leave-on considerations in Category 6 the Expert Panel for Fragrance Safety stated that the products in the already-mentioned category can be considered rinse-off with regard to phototoxicity.
Sub-Section 2.7 - Elimination of “Sunblock” Terminology in the Guidance:
Being considered an outdated definition, “sunblock” has been substituted by the term “UV Filters”.
Sub-Section 2.8 - Clarifications that make-up remover for face and eyes also includes potential exposure to lips.
Sub-Section 2.9 - Clarification about “aftershaves of all types” in Category 4:
In order to resolve a discrepancy present in Table 11 of the Guidance document, the wording “Aftershaves of all types” has been substituted by “Aftershaves of all types (except creams and balms)”. Moreover, RMTF has also prevented the term “hydroalcoholic” from appearing due to the fact that there are also non-alcoholic products in the market.
Section 4: New IFRA Specification Standard
Furthermore, aside from what is already been discussed, IFRA introduced a grand total of 48 new standards, where 47 have been listed as new restrictions, while only 1 has been marked as a prohibition standard.
For the sake of this article’s fruition, the 48 changes will be listed in their entirety in a drop-down menu below this section and will be presented as intended in the 51st Amendment.
A Summary of the Revised IFRA Standards
Lastly, aside from the mentioned changes, IFRA also revised a number of pre-existing standards bringing forth a palette of modifications to the 51st Amendment that will be briefly recapped. Once again, for further information feel free to consult the link that was provided at the end of the premise.
For consistency and clarification purposes, IFRA decided to add a total of three new CAS Numbers. Two of the CAS Numbers have been added to Farnesol (16106-95-9 and 3879-60-5) in addition to its previous ones, while the remaining CAS Number has been associated to Mintlactone (38049-04-6). It is of pivotal importance to understand that these two updates do not affect the content of the Standard for the two molecules and, as such, the previously discussed implementation timeline is not bonding for Farnesol and Mintlactone.
Together with the revised restriction standards for Methyl-N-methylanthranilate and Tagates oil and absolute, IFRA specified that, as feedback from the Consultation, from now on Phototoxicity will also apply to Category 6, specifically to rinse-off products.
Based on systemic toxicity, IFRA has decided to revise the standards regarding Estragole and Methyl eugenol, reducing their limits.
The limitations applied to p-Cresol (1319-77-3 and 106-44-5) due to potential depigmentation do not extend to o-Cresol (95-48-7-) and m-Cresol (108-39-4).
Conclusion
Overall, IFRA’s 51st Amendment has brought numerous changes to the world of perfumery, with the sole intent of rendering the industry a safer, eco-friendly environment as a whole while, at the same time, trying to adjust the parameters already established in the previous amendments.
Although the Amendments are not bonding for every single perfumer in the world, except for IFRA’s members, it is relevant to state that adhering to such rules displays a concrete and conscious commitment to render the ever-changing world of perfumery a more transparent, safe, and friendly field; not only for the perfumers but for the consumers alike.
Once again, we from Scentspiracy would like to thank you for your time and we would like to consult the amendment directly from the IFRA website.
Written and redacted by Francesco Ramorino and Fulvio Ciccolo (Scentspiracy, 2023)
Sources:
Foto di Fulvio Ciccolo su Unsplash